thumbnail
Cardiovascular
Edwards to Spin Off Critical Care Business in 2024Edwards to Spin Off Critical Care Business in 2024
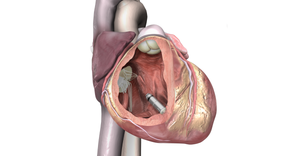
The Irvine, CA-based company said the measure will help it focus more on its structural heart offerings, which include transcatheter aortic valve replacement procedures.
Sign up for the QMED & MD+DI Daily newsletter.


.png?width=700&auto=webp&quality=80&disable=upscale)
.png?width=700&auto=webp&quality=80&disable=upscale)
.png?width=700&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)




















.png?width=300&auto=webp&quality=80&disable=upscale)
