Breast Cancer Detection Drug-Device System Nabs FDA Approval
The LumiSystem enables surgeons to scan the breast cavity post-lumpectomy in real time, detect, and resect residual cancer that otherwise could have been missed.
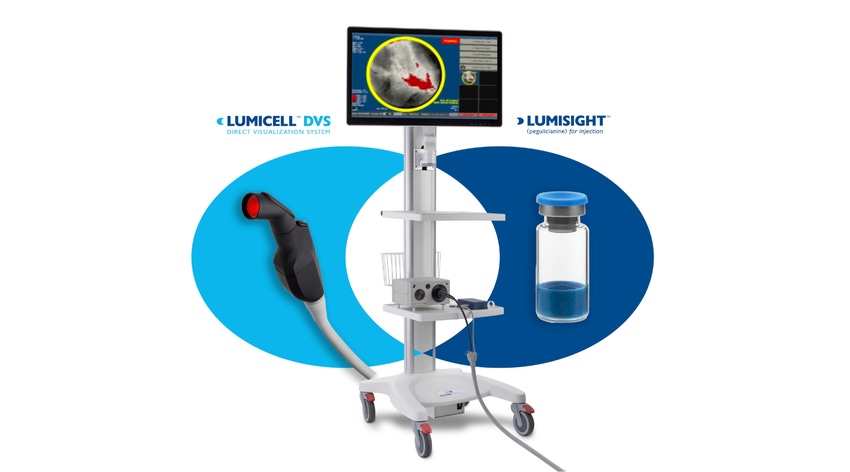
Recently, Lumicell, a company focused on the development of fluorescence-guided imaging technologies for cancerous tissue detection during surgery, announced FDA approval of its New Drug Application (NDA) for Limisight (pegulicianine) optical imaging agent and Premarket Approval (PMA) for Lumicell Direct Visualization System (DVS), together referred to as the LumiSystem. FDA approved the two products to assist in breast cancer detection within, “the resection cavity following removal of the primary specimen during lumpectomy surgery,” according to the agency.
The most common surgical procedure to treat breast cancer is lumpectomy. However, there are limitations to the surgery, including the difficulty of obtaining complete tumor resection during a single procedure. As many as 36% of patients reportedly need a second surgery to achieve clear margins and, “Up to 65% of the time, we do not find residual cancer in the second surgery and are left wondering if we performed an unnecessary surgery due to a false positive margin assessment or if the cancer was missed again in the second surgery,” said Irene Wapnir, MD, breast surgical oncologist and professor of surgery at Stanford University School of Medicine, in the company press release.
With the LumiSystem, according to Lumicell, surgeons are able to scan the breast cavity post-lumpectomy in real time, detect, and resect residual cancer that otherwise could have been missed. The combination of the agent and DVS is indicated for fluorescence imaging in adults with breast cancer as an adjunct for the intraoperative detection of cancerous tissue.
The safety and efficacy of Lumisight was evaluated in multi-center, intra-patient controlled clinical trail of patients with breast cancer undergoing lumpectomy and showed 84% diagnostic accuracy. Additionally, the safety of the system was established using data from more than 700 breast cancer patients across five studies, according to Lumicell.
The most common side effects when using Lumisight are hypersensitivity and abnormal urine color. Additionally, the company reported that Lumisight may cause serious hypersensitivity reactions, including anaphylaxis.
The approval comes after an FDA advisory committee, in a 16-2 vote, decided that the benefits of LumiSystem outweigh the risks. The march decision came with some caveats, with one panelist that voted yes saying the tool fell short of the achievement of no positive margins. Another panelist said that the benefits outweighed the small risk of anaphylaxis.
About the Author(s)
You May Also Like




