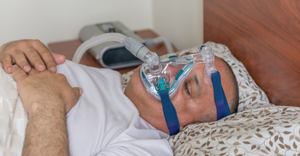
Thalidomide tablets collected by health officials in 1962 as part of an investigation. The drug was linked to deformities in
Regulatory & Quality
The Tragedy That Stalled Medical Device RegulationThe Tragedy That Stalled Medical Device Regulation
Trivia Tuesday: What global tragedy forced Congress to put medical device regulation on the back burner in 1962?
Sign up for the QMED & MD+DI Daily newsletter.