Medtronic Updates Investors on Key Product Pipelines
From Hugo to MiniMed and everything in between, here's the latest on several important Medtronic technologies.
August 24, 2022

Medtronic may be the largest publically-traded pure-play medical device company in the world, but not even Big Blue is immune from the macroeconomic challenges the rest of the industry is facing.
CEO Geoff Martha told investors during the company's fiscal first quarter (Q1 2023) earnings call that supply chain disruptions, inflation, hospital labor shortages, COVID-related procedure delays/cancellations, and foreign exchange factors – along with difficult comparisons to the prior year – caused revenue and earnings per share to decline.
"At the same time, there were several bright spots in the quarter across our businesses, including strength in pacing, cardiac surgery, U.S. core spine, neurovascular, diabetes in Europe, and strong overall growth in many emerging markets," Martha said, according to The Motley Fool's transcript of Medtronic's earnings call.
He also noted several near-term pipeline catalysts that are expected to accelerate growth.
TAVR is one of Medtronic's largest growth drivers
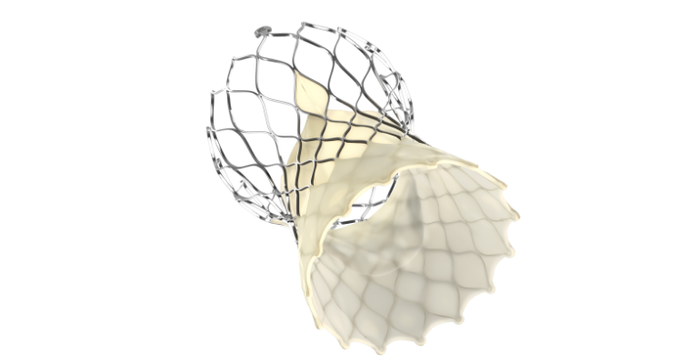
Martha said the limited U.S. market release of the Evolut FX transcatheter aortic valve replacement (TAVR) system (shown above) is receiving "an overwhelmingly positive customer reception."
The CEO said the Evolut FX enhances ease of use and provides implanters with greater precision and control during the procedure, and it maintains all of the hemodynamic and durability benefits of the Evolut platform.
"When you combine the FX launch in U.S., PRO+ launch in Europe and Evolut PRO launch in China, we feel really good about the opportunities in our TAVR franchise around the globe. TAVR is one of the largest growth drivers for Medtronic, and we expect the market, which is roughly $5.5 billion today, to exceed $7 billion within the next three years and reach $10 billion in the next five years."
Medtronic plans to disrupt the single-chamber ICD market
In cardiac rhythm management, Martha said Medtronic looks forward to disrupting the single-chamber ICD market with its Aurora extravascular implantable cardiac defibrillator (ICD).
"Now as you may know, one of our competitors has had a subcutaneous ICD in the market for many years," he said, most likely referring to Boston Scientific's Emblem S-ICD. "But it's remained a niche device given its limitations compared to conventional ICDs. With Aurora, we've created a true game changer where the electrophysiologist and the patient don't have to make trade-offs."
The device is expected to deliver the benefits of a traditional ICD, including the same size, battery longevity, and ability to use proven antitachy pacing in lieu of delivering a painful shock to terminate life-threatening arrhythmias.
"Aurora does all of this without having to place leads inside the heart," Martha said.
Medtronic plans to present EV-ICD global pivotal data this weekend at the European Society of Cardiology (ESC) Congress in Barcelona, Spain. The company is also awaiting CE mark approval for Aurora and expects FDA approval next calendar year, the CEO noted.
'Never Gonna Give You Up'
Despite earlier disappointments in renal denervation, Medtronic's persistance in the space may soon pay off.
Martha said the company is nearing completion of the six-month follow-up for the full cohort of patients in the company's SPYRAL HTN-ON MED study. Medtronic will then analyze the data and plan to present the findings in the next few months, he said. This data is expected to complete the final piece of the company's clinical module submission to FDA as every other module has been submitted, reviewed, and closed.
"The data on our Symplicity blood pressure procedure is robust, including strong pivotal trial results and compelling real-world registry data from over 3,000 patients," Martha said. "And more recently, data has been presented that show already in-patient spend nearly double the time and target blood pressure range through three years than those who received a sham procedure. This could have a profound effect on public health through the reduction of cardiovascular events, including stroke, heart failure, and [cardiovascular] mortality."
And after all of the company's trials and tribulations in renal denervation, it was no surprise to hear Martha say Medtronic expects to be a leader in this market, which the company projects to exceed $500 million by 2026 and $2 billion to $3 billion by 2030.
Medtronic is 'investing heavily' in surgical robotics
Medtronic continues to execute on the limited market release of its Hugo robot (shown below) Martha told investors during Monday's call. He said the company is installing new systems and collecting clinical data in approved geographies, enhancing the system based on surgeon feedback, improving supply chain resiliency, and scaling manufacturing production. Feedback and demand continue to be "very strong," he said.

Martha said the company has made progress over the last quarter, and is nearing the start of a U.S. investigational device exemption trial for a urology indication with the Hugo robot.
The company also continues to increase its user base of Touch Surgery Enterprise, its AI-powered surgical video and analytics platform. With Touch Surgery Enterprise, surgeons can now easily review film from their surgeries to continuously improve and advance patient care, Martha noted.
"Overall, when it comes to surgical robotics, we're investing heavily to become a major player in the market for the long term, leveraging our decades of experience and leadership in minimally invasive surgery," Martha said.
As Vijay Kumar, a medtech analyst at Evercore, pointed out during the Q&A portion of the earnings call, Martha's commentary on the Hugo robot carried a notable "change in tone" compared to the company's previous earnings call, with the CEO sounding much more bullish on the company's momentum in the surgical robotics space. That's largely because Medtronic has made significant progress on some of the supply chain concerns it previously had, and resolved some specific issues as well as its manufacturing capacity challenges, Martha explained. He also emphasized expanded sales in Europe.
"We had a number of installs and continue to get good feedback," Martha said. "And the surgeon feedback continues to be really strong. ... the word is out. They like the design. They like the approach we're taking to the market. And they realize that when we launch, we won't have all the indications yet, and we're going to be building out our instrument on the number of instruments we have with it, but they want to be part of this journey with us."
Closing the loop on pain
Martha also updated investors on the company's neuroscience portfolio, noting that it has submitted its Inceptiv evoked compound action potential (ECAP) closed-loop spinal cord stimulation (SCS) technology, designed to optimize pain relief for patients. He said the technology is expected to "revolutionize the SCS market."
Now turning to our neuroscience portfolio. In neuromodulation, we've submitted our inceptive ECAPs closed-loop stimulator. We expect inceptive's closed-loop therapy, which optimizes pain relief for patients to revolutionize the SCS market.
The company also continues to ramp commercial activities to go after the diabetic peripheral neuropathy (DPN) opportunity with its first cohort of DPN market development reps now trained, Martha said.
"We believe DPN is one of the largest opportunities in medtech, and we expect the market to reach $300 million by FY '26 with an annual total addressable market of up to $2 billion," he noted.
Progress on diabetes warning letter
In diabetes, Martha said Medtronic is in active dialogue with FDA on the company's regulatory submission for the MiniMed 780G with the Guardian 4 sensor, and "we remain focused on resolving our warning letter."
Late last year, FDA slapped Medtronic with a warning letter after an inspection that concluded in July 2021 related to a recall of the company's MiniMed 600 series insulin infusion pump, and a remote controller device for MiniMed 508 and Paradigm pumps. The warning letter focuses on the inadequacy of specific medical device quality system requirements at the company's Northridge, CA facility in the areas of risk assessment, corrective and preventive action, complaint handling, device recalls, and reporting of adverse events.
Martha said the company has completed more than 90% of the actions it committed to FDA.
In the company's continuous glucose monitoring (CGM) pipeline, Medtronic submitted its next-generation sensor, Simplera for CE mark.
"Simplera is disposable. It's easier to apply, and it's half the size of Guardian 4," Martha said. "The Simplera file is ready to submit to the U.S. FDA, and we're waiting to submit it as we're prioritizing the 780G Guardian 4 review. And with regards to our overall diabetes pipeline, we're making considerable investments well above our corporate R&D average."
About the Author(s)
You May Also Like




