Medtronic Growth Catalysts to Watch for in 2023
These products are expected to be strong growth drivers for the medical device industry's largest player.
February 22, 2023
This week during Medtronic's fiscal third quarter earnings call, CEO Geoff Martha highlighted several products in the company's portfolio and pipeline that are expected to move the needle this year.
Cardiac rhythm management
Martha said Medtronic continues to see strong market adoption of its leadless pacemakers, which grew 14% in the fiscal third quarter. The company also saw its defibrillation solutions business grow 7% and he highlighted the Aurora extravascular implantable cardioverter-defibrillator (EV-ICD) system, which just last week received a CE mark, allowing it to be marketed in the European Union. Medtronic designed the Aurora EV-ICD system to provide the life-saving benefits of traditional ICDs while avoiding certain risks because the lead is placed outside the heart and veins. The Aurora EV-ICD system is not yet available in the United States.
The Aurora EV-ICD is implanted below the left armpit and the Epsila EV lead is placed under the breastbone using a minimally invasive approach. Placing the lead outside of the heart and veins is designed to help avoid long-term complications associated with transvenous leads, such as vessel occlusion (narrowing, blockage or compression of a vein) and risks for blood infections.
Cranial and spinal technologies
Medtronic saw 6% growth in its core spine business and 8% growth in neurosurgery.
"This is driven by our market-leading ecosystem of Aible-enabling technology and the associated pull-through of our best-in-class spinal implants," Martha said, according to Seeking Alpha transcripts of the call. "From our AI-enabled surgical planning platform to our patient-specific and differentiated spine implants, to our imaging, our navigation, and robotic technologies, we are differentiating ourselves with spine surgeons around the world."
Surgical innovations (SI)
"Surgeons around the world prefer our advanced surgical products and we expect the momentum we are seeing in SI to continue," Martha said. "In particular, in our leading advanced energy franchise, we are seeing strong adoption of our recently launched cordless Sonicision 7 and we are preparing to launch our recently approved LigaSure XP."
Martha said these businesses contribute about 20% of Medtronic's revenue today and are collectively growing above the company average. Medtronic is investing disproportionately in these businesses and expect them to become a bigger part of the company's growth over time.
In structural heart, while the transcatheter aortic valve replacement (TAVR) market continued to be impacted by healthcare staffing challenges and COVID in Japan, Medtronic drove 11% growth in the fiscal third quarter, including 12% growth in the United States, Martha said.
"We are seeing great physician reception of our Evolut FX system, which just completed its full quarter of launch in the U.S.," Martha said. "Evolut FX combines industry leading durability with enhanced and predictable valve deployment. And in addition, data was presented during the quarter at PCR London Valves, showing Evolut FX’s Commissure alignment has improved significantly, which is important for coronary access and valve hemodynamics. Now looking ahead, we will continue to bring Evolut FX around the world and we are currently seeking approval in the Japanese and European markets."
A regulation-related headwind in China hampered growth in Medtronic's cardiac ablation solutions business, which grew just 3% in the fiscal quarter. However, Martha said the business is seeing growth outside of China in the high single-digits, including low double-digit growth in the United States on the continued strong adoption of the company's Arctic Front cryoablation technology.
"We are also advancing what we believe will become the leading pulsed field ablation portfolio," Martha said. "And in two weeks, highly anticipated data for our PULSED AF pivotal trial will be released in the late-breaker session at ACC. The trial is evaluating our Pulse Select PFA catheter in both paroxysmal and persistent patients and this will be the first results from an [investigational device exemption (IDE)] trial in the PFA space and we are on track to be one of the first companies with a PFA catheter in the U.S. market."
The CEO said the company is also working to bring its Affera mapping and navigation platform and Sphere-9 catheter to market as Medtronic completed enrollment in a pivotal trial during the quarter.
"Sphere-9 can perform high-density mapping and deliver either PFA or RF energy, all from the same catheter," Martha said. "And given Sphere-9 is a focal point PFA catheter, it is highly complementary to our Pulse Select anatomical PFA catheter."
The company also just launched the AcQCross transseptal access system, which was among the products that Medtronic bought last year from Acutus Medical. Martha said it is the only system approved for both mechanical and RF crossings.
"So when you think about our Arctic Front crysolution, our DiamondTemp RF catheter, our PFA catheters, our left heart access solutions and our Affera map nav system, we are assembling a leading ecosystem of technologies to meaningfully increase our participation in the fast growth $8 billion EP ablation space," Martha said.
Medtronic acquired Affera last year for an undisclosed sum.
Surgical robotics
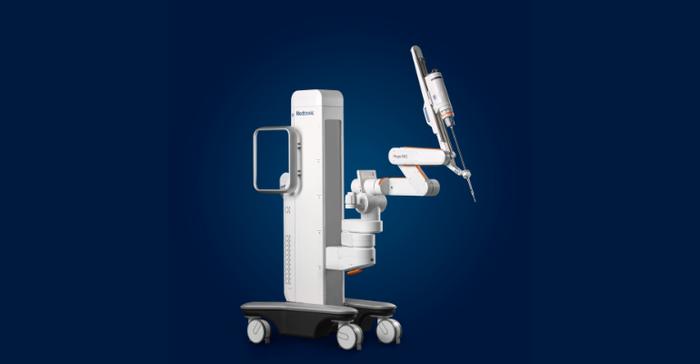
"In surgical robotics, we are making good progress as the second major player in this exciting space," Martha said. "We continue to see positive sales momentum with the rollout of our differentiated Hugo robotic system in many international markets, and we started our U.S. IDE trial for our urology indication during the quarter. Given less than 5% of surgical procedures globally are done robotically, we expect our surgical robotics business to become a meaningful growth driver for Medtronic."
The competitive landscape of the robotic surgery market has been heating up for a years now, but when the dust settles, the soft-tissue robotics field will end up with between three to five major surgical robotics systems, according to Alex Mottrie, MD, a pioneer in the field of robotic surgery. It's becoming increasingly clear that the top of the robotic surgery food chain will be Intuitive Surgical and Medtronic. Mottrie, who has performed more than 4,000 robotic surgery procedures since 2001 and was the first surgeon to perform a prostatectomy using the Medtronic Hugo Robotic Assisted Surgery System last year, shared some candid insights last year on the current robotic surgery landscape during a call hosted by medtech analysts at BTIG. From a 30,000-foot view, the key differentiator between Medtronic's Hugo and Intuitive Surgical's da Vinci platform seems to come down to procedure type, according to analyst takeaways from that call. Currently, both systems are best suited for urological and gynecological procedures, however Hugo's four independent arms could eventually increase interest in using the system for general surgery and colorectal surgery, as the design allows for better abdominal access, according to Mottrie.
Neurovascular
Medtronic's neurovascular business managed to grow 9% in the fiscal third quarter despite being impacted by the same China headwind that impacted some of the company's other businesses. While Martha did not call attention to any specific neurovascular products, he said the business continues to see strong growth in several categories, including flow diversion, aspiration, and stent retrievers.
"Given stroke is the number two cause of death globally and there is still very low therapy penetration, we see a long runway for high growth in this market that is approaching $4 billion," Martha said.
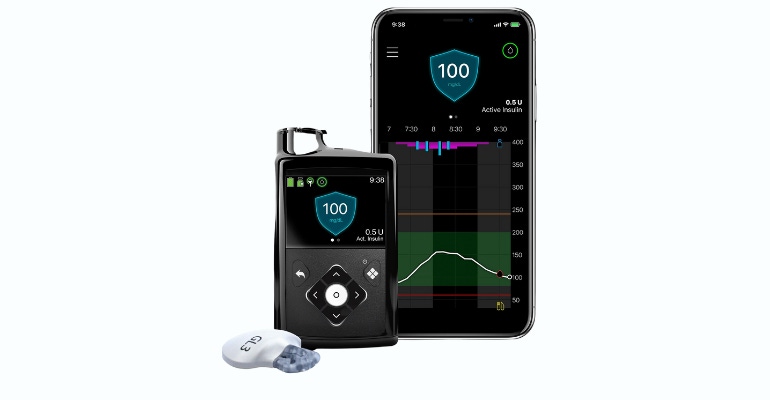
Diabetes
The company is seeing strong international growth in diabetes, Martha said, but this growth is offset by declines in the U.S. market where Medtronic was hit with an FDA warning letter in December 2021. That warning letter stemmed from an inspection that concluded in July 2021 related to recalls of the MiniMed 600 series insulin infusion pump, and a remote controller device for MiniMed 508 and Paradigm pumps.
"We remain focused on resolving our FDA warning letter and are ready for reinspection," Martha said. "We also remain in active review with the FDA on our submission of the MiniMed 780G system with the Guardian 4 sensor."
Outside the U.S., the company's diabetes business grew 18% on continued strong sales momentum of the MiniMed 780G and Guardian 4 sensor, Martha noted. He said the 780G is now launched in more than 90 countries, up from 60 last quarter.
"We continue to invest heavily in assembling our ecosystem of durable pumps, smart pens, patch pumps, sensors, algorithms, and customer service with multiple programs under development, all with the intent of restoring strong growth of our important diabetes franchise over the coming years," Martha said.
Other Medtronic businesses
Medtronic also reported double-digit growth in cardiac diagnostics as the company ramped up production of its Linq II insertable cardiac monitor.

Martha also noted that the company's GI business grew in the high single digits on strong adoption of GI Genius, which uses artificial intelligence to help physicians detect polyps during colonoscopies. Last year, the GI Genius endoscopy module was named to the prestigious Fortune 2022 "Change the World" list. It is the first commercially available computer-aided detection system that uses AI to help physicians identify polyps and flag signs of colon cancer that might otherwise be missed.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
