Stryker executives say Mako continues to be the robotic-assisted choice for hip and knee replacements, but installations of the robot were nonetheless soft in the third quarter.
November 8, 2022
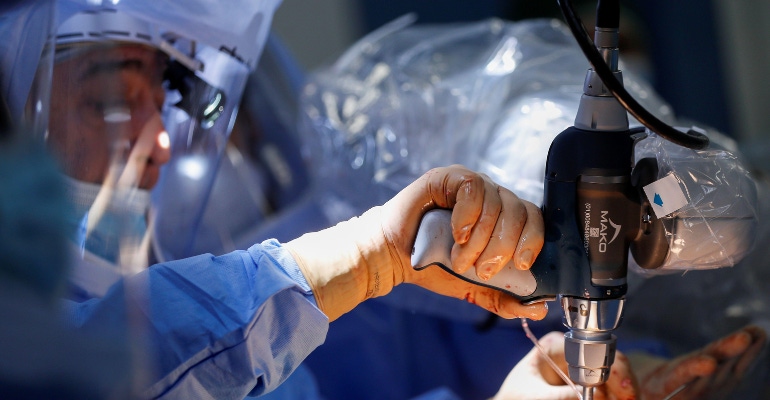
Analysts balked at the price Stryker paid for Mako back in 2013 – $1.65 billion – but the company has proven time and again that the juice was worth the squeeze. Even with the unpredictability of the past two years, Stryker's surgical robotics system has often been a saving grace in the face of pandemic-related headwinds.
Stryker stopped short of providing specific Mako earnings metrics during the company's most recent earnings call, but executives did say Mako installations for the third quarter were soft due to delays stemming from variability in the hospital environment. This appears to be yet another example of how hospital labor shortages are impacting medical device companies.
Despite the softness in installations, Mako continues to be the "robotic-assisted choice in the marketplace" for hip and knee replacements, said Jason Beach, Stryker's vice president of investor relations. He also said the robot's order book remains strong and the company expects a good fourth quarter for Mako.
Stryker CEO Kevin Lobo said he is encouraged by the fact that Mako as a percentage of knees and hips continues to go up quarter after quarter.
"So, all those vectors are heading the right direction," Lobo said. "Yes, we've had some more delays in getting these robots installed and sold to our customers. But Q4 will be good and will continue our positive trajectory."
Stryker expects to update investors on key Mako metrics in January.
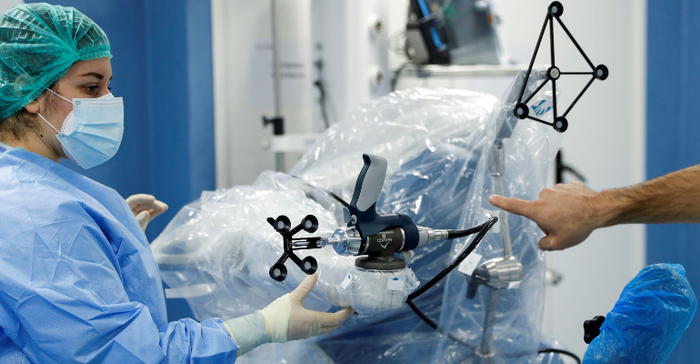
Operating room staff prepare a Mako robotic-assisted surgery system before a knee surgery at the UPMC Salvator Mundi International Hospital in Rome, Italy, February 5, 2021.
The executives also reported that the Mako spine indication moved ahead in its development while the Mako shoulder indication moved back a step due to a change in the associated shoulder implant.
"We were on a certain timeline with Stryker shoulder implants, but we wanted to switch to the Tornier implants. They're the market-leading implants. So, we had to sort of delay that one," Lobo said. "But Spine has been moved forward."
Mako gives Stryker a 'giant advantage' in the ASC trend
As routine procedures like hip and knee replacements continue to move out of the hospital and into ambulatory surgery centers (ASCs), Stryker is playing offense to get into those ASCs.
"Our offense continues to exceed our expectation," Lobo said during the third-quarter earnings call. "Just the bundle that we provide for the ASC to make life easy for them has been very well received. Close to half of the deals that we win includes a Mako in these orthopedic ASCs. We've now crossed sort of the 10% mark of large joints that are done in ASCs."
Lobo said the company's growth in the ASC setting isn't going up at a rapid rate, but it is a "steady climb."
The gating factor, as Lobo puts it, is simply construction and refurbishment of the centers to enable more procedures.
"But every hospital system I talked to is preparing themselves for another ... a new ASC," Lobo said. "It's a trend that had started prior to COVID and has accelerated. And we expect that, that will continue. But we love our position. The breadth of our portfolio, having capital disposables and implants puts us in a really leading position. And Makos, as I said, almost all the time are included, and that gives us a giant advantage."
About the Author(s)
You May Also Like




