Abiomed’s Impella Now Linked to 4 Deaths in Recent Recall
With many companies feeling the sting of device recalls in recent months, Abiomed’s second Impella recall in 2023 has been updated to include heartbreaking new stats.
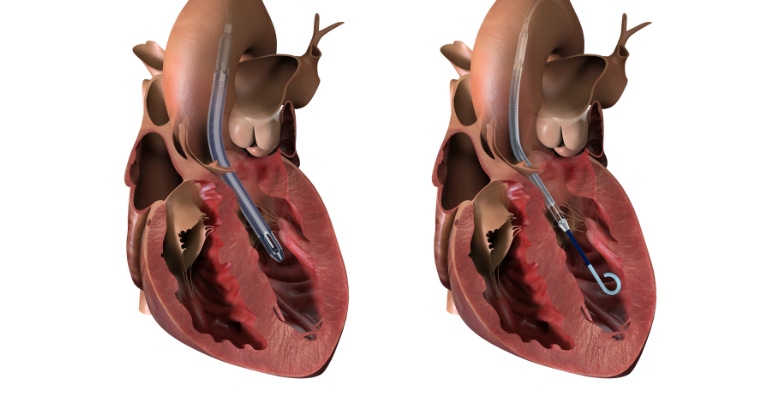
Adding another layer to Abiomed’s challenging last few months connected to its Impella heart pumps, FDA has updated the device’s Class I recall regarding a potential conflict in patients with transcatheter aortic valve replacements (TAVR), adding that four deaths have now been linked to the issue.
At the time of the recall announcement, Johnson & Johnson (J&J) told MD+DI that “on June 14, we issued a notification to customers regarding the safe use of left sided Impella heart pumps in patients with a [TAVR] valve. The notification addresses the potential risk for unintentional interaction of the Impella motor housing with the distal stent of a previously implanted TAVR valve and provides further recommendations on how to position Impella in these patients.”
The potential interaction includes collision of the stentlike struts along the outer edges of the TAVR with Impella’s spinning impeller, which can shear the impeller blades and cause parts of the pump to fracture. Not only can the interaction spread debris into the bloodstream, but a collision may also result in a dangerous loss of blood flow through the damaged Impella device.
Originally, the notice identified 27 complaints, which the J&J division said is about 0.7% of all patients with TAVR’s treated with Impella since 2016. FDA then posted a notice July 27 updating the number to 30 complaints, including 26 injuries and four deaths.
The notice said the company is recalling the pumps because its instructions for use do not “adequately address precautions to take when treating patients who have undergone [TAVR]. The [instructions for use] lacks guidance to clinicians on how to manage use of Impella in patients with TAVR and fails to describe how the issue may present if an Impella interacts with TAVR.”
The device is not being removed from hospitals and doesn’t need to be returned to the manufacture. Additionally, Abiomed is currently updating the device instructions.
“This notification was not a device removal,” Abiomed wrote in an email to MD+DI at the time of the initial recall. “These devices remain available, and Impella technology can continue to be used safely in patients with a previously implanted TAVR valve.”
Abiomed is far from the only company recently caught in the crosshairs of an FDA recall.
In June, Philips was hit with a Class I recall of its Triliogy Evo Ventilator due to extended environmental contamination exposure potentially affecting the devices air path. That same month, Teleflex recalled its Rüsch endotracheal tubes after reports of disconnection between the 15mm connector from the endotracheal tube potentially causing oxygen desaturation.
In July, J&J’s Ethicon recalled several types of Megadyne electrodes due to risk of serious burn injuries to patients, Draeger recalled the Oxylog 3000 Plus Emergency and Transport Ventilator after reports the battery died even after being re-connected to AC power, and Integra initiated a global recall of all products manufactured in the company's Boston, MA facility, noting that the company expects the recall to extend into 2024.
Now in the starting stages in August, Baxter announced a recall of its Sigma Spectrum Infusion Pump with Master Drug Library (version eight) and Spectrum IQ Infusion System with Dose IQ Safety Software (version nine) after seeing increased false alarms for upstream occlusion after upgrading the software to version v8.01.01 and v9.02.01.
Additionally, only a few days ago, Abbott announced it was throwing in the trifecta towel, reporting it has permanently withdrawn the devices from the market after FDA raised durability concerns in early 2023.
About the Author(s)
You May Also Like




