2020 Was a Record Year for Novel Medical Devices
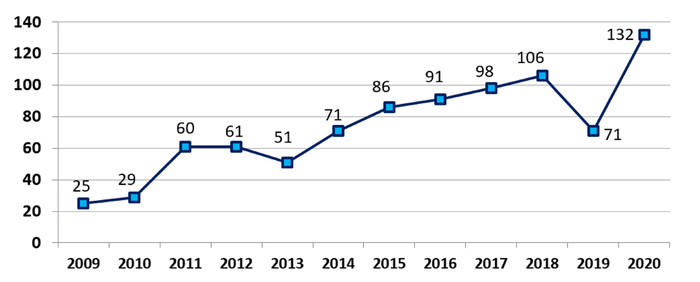
FDA approved, cleared, or authorized a record high of 132 novel medical devices in 2020, surpassing the 40-year high set in 2017.
February 17, 2021
Despite the challenges brought on by the COVID-19 pandemic, 2020 was a record year for novel medical devices, FDA said Monday.
Jeff Shuren, MD, director of FDA's Center for Devices and Radiological Health (CDRH), said the agency approved, cleared, or authorized a record high of 132 novel medical devices last year, surpassing the 40-year high mark set in 2017 and capping off 10 years of progress.
"It is a big leap from the 29 novel devices we authorized a decade ago, in 2010," Shuren wrote in a message to FDA stakeholders.
Novel devices include those brought to market through the premarket approval (PMA), humanitarian device exemption (HDE), and de novo pathways, as well as a subset of those that are brought to market with 510(k) clearance or emergency use authorization (EUA). Of the thousands of 510(k) clearances every year, FDA considers only those devices with a breakthrough designation to be novel. Shuren said that the 2020 number of novel medical devices also includes first-of-a-kind devices authorized under an EUA.
"Novel or innovative does not simply mean 'new.' They address an unmet need, or may be safer or more effective than currently available alternatives," Shuren said. "For FDA-approved and FDA-cleared medical devices, innovation and safety are two sides of the same coin."
The high number of novel medical devices that received a greenlight from FDA in 2020 underscores the importance of medical device manufacturers engaging with FDA reviewers early and often in the development process, which is something CDRH has been encouraging manufacturers to do for some time now.
Novel Medical Devices Authorized Per Calendar Year 2009 - 2020
COVID-19 played a big role in novel medical devices record
While the number of novel devices seen in 2020 cannot be attributed to any single factor, Shuren acknowledged that the volume of EUAs for novel devices issued in 2020 played a role. Of the 625 EUAs the FDA issued for medical devices in 2020, such as tests and sample collection devices, personal protective equipment, ventilators, and other types of devices brought to market in response to COVID-19, several were issued for novel medical devices.
One example was the first sample pooling in diagnostic testing for COVID-19, an EUA granted to Quest Diagnostics in July 2020. Another example was LabCorp's RT-PCR test, which was granted expanded indications to include asymptomatic people, or those without known or suspected COVID-19 infection. FDA reissued the LabCorp EUA to include the new indications in July 2020.
Qiagen also secured an EUA for test included in the record number of novel medical devices in 2020. In March 2020, the testing company brought to market the first combination diagnostic test for the detection and differentiation of the viruses that cause flu and COVID-19. Davide Manissero, MD, chief medical officer of infection and immune diagnostics at Qiagen, chatted with MD+DI Managing Editor Omar Ford during a recent episode of our Let's Talk Medtech podcast.
"These examples demonstrate the remarkable ability of the device ecosystem and the FDA to adapt and respond to emerging public health needs," Shuren said.
Novel medical devices in 2020 that had nothing to do with COVID-19
While COVID-19 certainly played a role in making 2020 a record year for novel medical devices, the vast majority of novel devices brought to market last year had nothing to do with the pandemic. For example, Guardant Health brought to market the first liquid biopsy next-generation sequencing companion diagnostic test.
Caption Health also scored a nod in 2020 for the first cardiac ultrasound software that uses artificial intelligence to guide the user to capture quality diagnostic images. FDA authorized the software for marketing in February 2020, and later in the year Caption Health turned its attention to developing an AI-guided lung ultrasound.
Other novel devices authorized in 2020 include:
The first game-based digital therapeutic to improve attention function in children with attention deficit hyperactivity disorder
An anterior cruciate ligament (ACL) implant as an alternative to ACL reconstruction to treat ACL tears
The first continuous renal replacement therapy device for a lower weight pediatric population with sudden or temporary loss of kidney function or fluid overload
The first-of-its-kind automated insulin delivery and monitoring system for use in young pediatric patients
A high density lipoprotein plasma delipidation system to reduce coronary artery atheroma in certain adult patients
A high intensity focused ultrasound (HIFU), magnetic-resonance guided, ablation system for the treatment of osteoid osteomas, a type of tumor, in the extremities
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
