Exo Iris Lung and Cardiac AI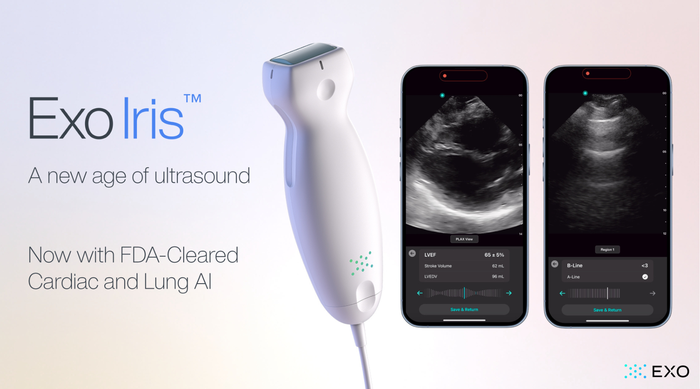
Artificial Intelligence
Exo’s FDA Cleared Cardiac & Lung AI Now on Iris Handheld UltrasoundExo’s FDA Cleared Cardiac & Lung AI Now on Iris Handheld Ultrasound
With the newly approved applications, Exo is now cleared for cardiac, lung, bladder, hip, and thyroid.
Sign up for the QMED & MD+DI Daily newsletter.








.png?width=300&auto=webp&quality=80&disable=upscale)


.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)





.png?width=300&auto=webp&quality=80&disable=upscale)




.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)



.png?width=300&auto=webp&quality=80&disable=upscale)


.jpg?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)