Eko Health Nabs FDA Clearance for Low EF Detection AI
The tool will enable ejection fraction detection quickly and accessibly using an Eko stethoscope during a routine physical exam.
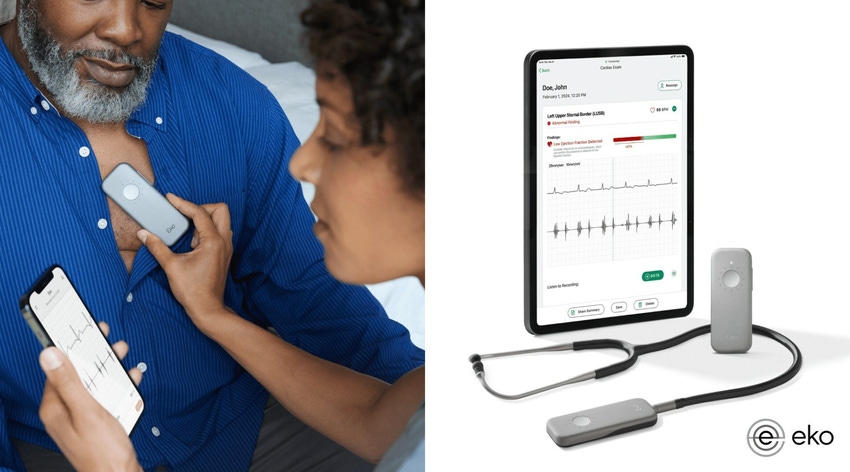
Eko Health, a digital health company, today announced the FDA clearance of its Low EF detection AI. With the clearance, low ejection fraction (EF) can be detected in 15 seconds using an Eko stethoscope during a routine physical exam.
EF is a key indicator of heart failure and is measured as a percentage of the total amount of blood in the heart that is pumping out with each heartbeat, according to Penn Medicine. A normal EF is 50% or higher. When EF is below 40%, the heart isn’t pumping enough blood and may be failing.
Traditional heart failure tools, like echocardiography, are often unavailable in the primary care setting due to cost, the specialized training required, and significant time added to seeing patients. The Low EF AI, however, embeds “rapid and accessible low [EF] detection into a stethoscope exam on the front lines of care,” according to the press release announcing the clearance.
"The stethoscope, the most recognizable symbol of healthcare, touches the lives of an estimated one billion people around the globe every year," said Connor Landgraf, co-founder & CEO of Eko Health, in the release. "With Eko's Low EF AI, we've transformed the icon of medicine into an AI-powered heart failure early detection tool that can help improve access to care for millions of patients, at a fraction of the time and cost of echocardiography."
During the clinical development of the artificial intelligence (AI) tool, Low EF was trained on a proprietary dataset of over 100,000 ECGs and echocardiogram pairs from patients. Additionally, it was clinically validated in a multi-site, prospective clinical study of 3,456 patients and received an “AUROC of 0.835 for detection of LVEF <40%, 74.7% sensitivity and 77.5% specificity, demonstrating a strong ability to differentiate between low and normal EF,” according to the company.
An independent validation of the AI took place at the Imperial College London, receiving scores as follows: AUROC of 0.85 for detection of LVEF below 40%, 84.8% sensitivity, and 69.5% specificity when deployed on over 1,050 patients across multiple real-world settings. In response to the findings, the UK NHS and Imperial College London extended Eko’s deployment to over 100 London and Wales clinics.
The tool was studied for its impact on pregnant people in Nigeria and saw the identification of twice as many cases of pregnancy-related cardiopathy than standard care. The statistics included an AUROC of 0.98, 100.0% sensitivity, and 79.4% specificity.
Eko also announced that the AI tool will also be added to the company’s Sensora Cardiac Early Detection Platform which has FDA-cleared algorithms to identify AFib and structural heart murmurs.
About the Author(s)
You May Also Like




.png?width=300&auto=webp&quality=80&disable=upscale)