Automated Artificial Urinary Sphincter Implant Update
UroMems reported an important update on its automated artificial urinary sphincter.
December 14, 2023
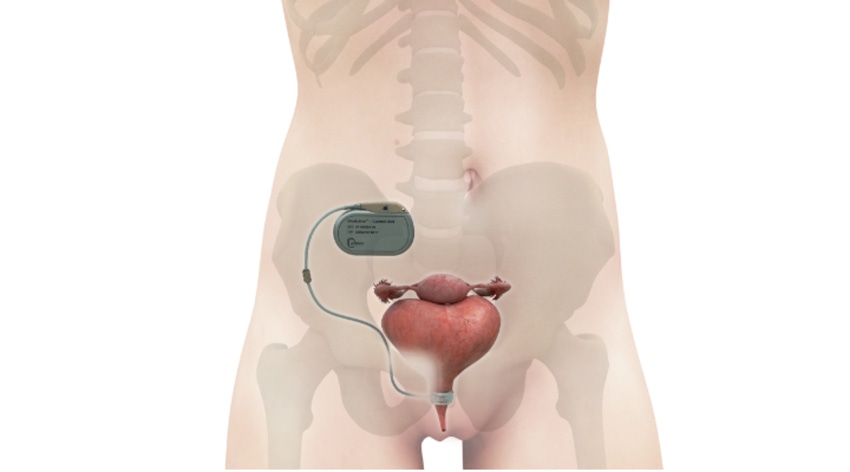
Uromems, the company developing an automated artificial urinary sphincter implant for both men and women with stress urinary incontinence (SUI), hit a significant milestone. The Grenoble, France-based company reported the complete treatment cohort in its clinical feasibility study has successfully reached the six-month primary endpoints.
The feasibility assessment of the UroActive System was completed through a prospective multicenter study. UroActive is the first automated artificial urinary sphincter (AUS) to treat stress urinary incontinence. The results of this study support design and implementation of UroMems’ pivotal SUI trial in Europe and the United States.
All six men enrolled in the study have now had the implant for at least seven months and up to 15 months, with their devices operating as expected and no need for revision nor explant. In addition, the company noted positive follow-up was received on secondary outcomes measures, including leak rate values and patient quality of life questionnaires.
“We’re so pleased to see that our expectations about our device’s performance were met or even exceeded and delighted to successfully treat and receive such high praise from patients who had been suffering from SUI for years,” said Pierre Mozer, the company's co-founder and chief medical officer. “The results of this study will allow us to prepare our pivotal study which will be a major step in the development of UroActive.”
UroActive is powered by a MyoElectroMechanical System (MEMS) that is placed around the urethral duct and is adjustable based on the patient’s activity. It is based on a unique bionic platform using embedded smart, digital, and robotic systems which, based on data collected from a patient, create a treatment algorithm that is specific for each patient's needs.
“The very compelling results of this first-in-man clinical study demonstrate the high potential of our technology and pave the way for larger clinical trials that will allow us to demonstrate all the benefits we are expecting to offer patients suffering from debilitating SUI,” said Hamid Lamraoui, the company's other co-founder and CEO. “We could not have reached this important milestone without the enthusiastic participation of the men in this initial study cohort. We’re extremely grateful to them for being a vital part in bringing this potentially revolutionary treatment to market.”
Stress urinary incontinence, or involuntary urinary leakage, affects an estimated 40 million Americans and 90 million Europeans, and occurs when the pressure in the bladder exceeds that of the muscle (the sphincter) around the urethra, caused by activities involving high intra-abdominal pressure, like coughing, laughing, and exercising.
Earlier this year, a woman in France became the first female to receive the automated artificial sphincter device for the treatment of stress urinary incontinence. The first male patient to receive the device was implanted last year.
Behind the Design
To help inform the design of the device, the UroMems team studied the existing device, namely the mechanical artificial sphincter, to learn what surgeons and patients liked about the device and what they found lacking.
The predicate device is a hydraulic device that consists of three parts: a cuff that wraps around the urethra to control the flow of urine; a balloon, which is placed under the abdominal muscles and holds the same fluid as the cuff (this is where the fluid is moved to when the cuff is open or deflated); and a pump, which relaxes the cuff by moving the fluid from the cuff to the balloon. The pump is placed in a man's scrotum or within the major labia in a woman. Artificial sphincter users must squeeze the pump through the skin to urinate.
Lamraoui said the company has a house rule that all the constraints are for the engineers – the device must be simple for the patients.
"It's a rule that we have in house, and we will never give up on this," he told MD+DI earlier this year.
So, it didn't take the UroMems team long to determine that the new device should not require patients to manually open the cuff by squeezing the pump through the skin.
"The issue with the balloon is the fact that it does not always generate the adequate pressure," he said.
But the physician selects how much pressure the balloon will generate during the procedure, and Lamraoui said there's no real rationale behind how much pressure the physician selects. If the pressure is too low, the patients will still have leaks, he said. But if the pressure is too high, the patient will be happy at first, but after a few years they will develop atrophy because the pressure is too high.
So, in designing the new device, the UroMems team decided to remove both the pump and the balloon, but they kept the urethral cuff. Instead of a pump and balloon system, the company developed a pacemaker-like control unit to automate the artificial sphincter device, as shown in the illustration below of the device implanted in a female anatomy.
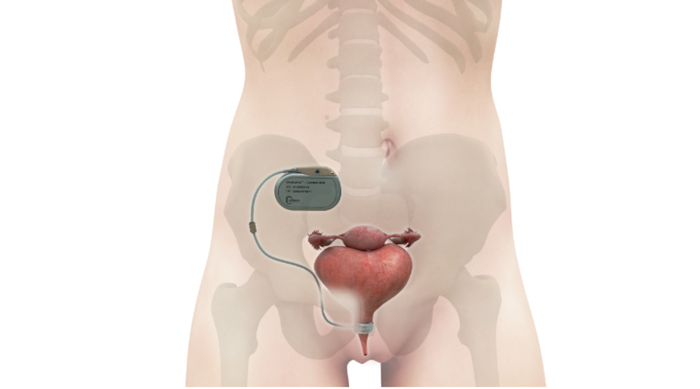
Image courtesy of UroMems
When asked to share a big-picture lesson that could be applied to other complex medical device projects that peers may be working on, Lamraoui said physician-engineer collaboration is key.
“The success so far is based on the fact that we work every day with physicians,” he said. “We have weekly meetings with surgeons.”
These meetings include at least two surgeons, sometimes more, who ask questions of the engineers and help to define user requirements and offer feedback on the development.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
