August 23, 2023

Medtronic's diabetes business was a sweet spot in the company's fiscal first quarter of 2024.
After FDA approved the company's MiniMed 780G system with the Guardian 4 sensor in April, analysts and investors wondered if the approval would be enough to move the needle for Medtronic's diabetes business. It certainly seems like it has.
CEO Geoff Martha touted "very strong demand" for the MiniMed 780G system in markets around the world.
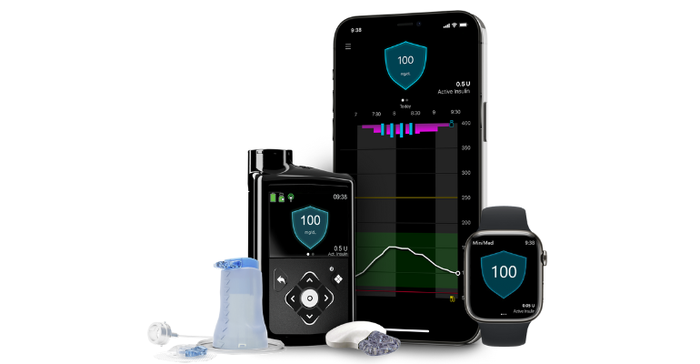
Available in Europe since 2020, this new system is designed with meal detection technology that provides automatic adjustments and corrections to sugar levels every five minutes for both basal (background) and bolus (mealtime) insulin needs.
Medtronic originally submitted for FDA approval of the MiniMed 780G in 2021, and received a regulatory license from Health Canada in 2022. But FDA approval was delayed by quality control problems at the company's manufacturing facility, which triggered a warning letter for Medtronic's diabetes unit. That warning letter stemmed from an inspection that concluded in July 2021 related to recalls of the MiniMed 600 series insulin infusion pump, and a remote controller device for MiniMed 508 and Paradigm pumps.
"We're getting great feedback that users are feeling a difference within one to two days," Martha said during Tuesday's earnings call, according to Seeking Alpha transcripts. "And our real-world evidence indicates that 90% of users are achieving or exceeding their glycemic targets when using our recommended settings as well as getting burden reduction in their diabetes management. And this differentiated value proposition is showing up in our results."
In developed markets outside the United States, Medtronic's diabetes business grew 18%, the highest growth for that business in four years. Martha attributed the growth to both 780G adoption and increased continuous glucose monitoring (CGM) attachment rates. In the United States, the 780G launch drove "low 30s growth" in U.S. durable pump sales for the diabetes unit. Since late June, the company has seen a 30% increase in unique prescribers with now more than 13,000 since the device launched in the United States. The CEO also noted that more than half of the 770G install base has upgraded or placed an order for the 780G since it launched.
Martha said demand is coming not only from the company's existing installed base, but that Medtronic is also getting competitive conversions.

According to Medtronic, the MiniMed 780G system features the lowest glucose target setting (as low as 100 mg/dL) in any automated insulin pump on the market and one that more closely mirrors the average glucose of someone not living with diabetes. With this setting, the pump will "treat to target" and will automatically deliver basal insulin adjustments and autocorrections to a set target, the company said.
It's also the only pump with an infusion set that can be worn for up to seven days, doubling wear time with advanced materials that help reduce insulin preservative loss, maintain insulin flow and stability, resulting in a reduced risk of infusion set occlusion, Medtronic noted. Combined with the Guardian 4 sensor requiring no finger sticks with SmartGuard technology, the MiniMed 780G system delivers a user-friendly design, the company said, adding that 94% of users report they're satisfied with the impact the system has on their quality of life. Importantly, users also reported remaining in SmartGuard technology 95% of the time, Medtronic said.
"So, the turnaround in diabetes is real and underway, and I am pleased with the progress the team is making," Martha said. "And we're just at the beginning of this inflection point for the business.
The company sees the intensive insulin space moving from primarily standalone CGM today to one that is smart dosing through either an automated insulin delivery (AID) systems or smart MDI (multiple daily injections), and Medtronic's diabetes business is well positioned for this trend, Martha said.
The company continues to invest heavily in next-generation durable pumps, smart pens, patch pumps, sensors, and algorithms, Martha said, and the business has multiple programs under development.
Que Dallara, EVP and president of Medtronic's diabetes business, said the business has had the highest new patient growth in three years and that's coming from MDI patients as well as competitive switches.
Medtronic also has a next-generation CGM under FDA review.
"It's very difficult to put a precise timeline on when we expect to have approval, but that is progressing," Dallara said during the earnings call.
How will new weight-loss drugs impact Medtronic's diabetes business?
Analysts were interested in hearing the company's leadership comment on noise around GLP-1, a new class of weight-loss drugs that some say could have wide-reaching impacts on medtech.
"First of all, I'd say it's an important class of drugs and like you, we're monitoring to see how the GLP-1 therapy is being adopted," Martha said. "That said, our initial work indicates minimal impact to our business. ... The one area we've seen some modest impact is bariatric, but this is really a small impact and this is a relatively small part of our surgery business. We actually operate obesity clinics in Europe as well. And they're talking about, longer term, this could actually bring in the bariatric space more patients into the funnel. Short term, we've seen some impact, again, modest and bariatric is a relatively small part of our surgery business. Longer term, we'll see how it plays out. There's a bit of optimism there though."
Importantly, the CEO said the company does not anticipate any GLP-1 impact in the type 1 diabetes population, which is the vast majority of Medtronic's diabetes business.
"And this is overall, I just don't see GLP-1s having a material impact on our business and medical device therapies at large. That's what our work is showing us at this point."
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
