Edwards Wins FDA Approval for TTVR Way Ahead of Schedule
The transcatheter aortic valve replacement (TAVR) pioneer is leading the way again – this time in the transcatheter tricuspid valve replacement (TTVR) market.
February 2, 2024
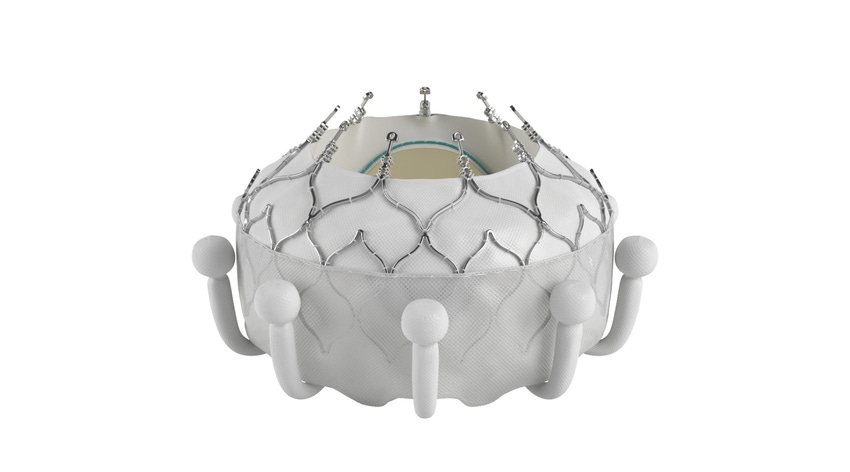
The company that pioneered the transcatheter aortic valve replacement (TAVR) market is leading the way again, this time in the transcatheter tricuspid valve replacement (TTVR) market.
Edwards Lifesciences has secured FDA approval for its Evoque TTVR system for the treatment of eligible patients with tricuspid regurgitation (TR). The Irvine, CA-based company had previously said it didn't expect FDA approval until at least mid-year, if not closer to the end of 2024. Edwards did, however, receive a CE mark for the Evoque TTVR system in October, making it the world's first transcatheter valve replacement therapy to receive regulatory approval to treat TR.
“Edwards has a long history of leading innovation and pioneering new therapies to address the unmet needs of patients with structural heart disease,” said Daveen Chopra, Edwards’ corporate vice president of transcatheter mitral and tricuspid therapies. “We are grateful for the strong collaboration with clinicians all over the world who contributed to the Evoque system now being available through FDA’s breakthrough pathway to provide a treatment option to the many patients in the U.S. suffering with tricuspid valve disease.”
The Evoque system is comprised of a nitinol self-expanding frame, an intra-annular sealing skirt, and tissue leaflets made from the company’s proven bovine pericardial tissue. The Evoque valve will be available in three sizes, all delivered through the same low-profile transfemoral 28F system.
Evoque is indicated for "the improvement of health status in patients with symptomatic severe TR despite optimal medical therapy (OMT), for whom tricuspid valve replacement is deemed appropriate by a heart team."
Successful six-month results from the randomized controlled pivotal trial, TRISCEND II, were presented at the Transcatheter Cardiovascular Therapeutics meeting last year (TCT 2023). The investigators presented favorable safety and effectiveness outcomes, demonstrating superiority to OMT alone, and the study met all primary endpoints. Key findings in the trial included a significant reduction or elimination of tricuspid regurgitation and significant and sustained quality of life improvement, while demonstrating a favorable balance between risk and benefit.
In addition to the six-month cohort, 318 of the total 392 randomized patients completed a one-year visit. The results showed favorable trends in the device group compared to the control group. Edwards expects to present the full cohort of 392 TRISCEND II pivotal trial patients at TCT 2024 in late October.
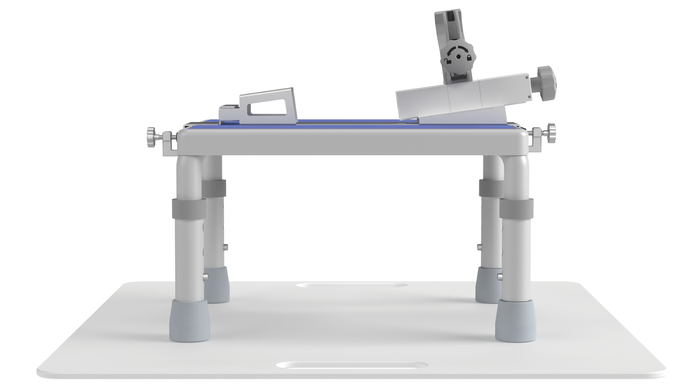
Evoque TTVR delivery system (stabilizer, base, and plate). IMAGE COURTESY OF EDWARDS LIFESCIENCES
“Patients suffering with tricuspid regurgitation endure life-impairing symptoms and, until today, had no approved transcatheter treatment options,” said Susheel Kodali, MD, director of the Structural Heart and Valve Center at Columbia University Irving Medical Center/New York-Presbyterian Hospital and principal investigator for the TRISCEND II study. “The Evoque system is able to replace the native tricuspid valve, virtually eliminating tricuspid regurgitation in a wide range of patients. We see significant improvements in patients’ symptoms and quality-of-life, including not feeling short of breath and being able to care for themselves, which ranked highest on a patient preference survey conducted at baseline with TRISCEND II pivotal trial patients.”
Will the TAVR playbook work in TTVR?
Robbie Marcus, a medtech analyst at J.P. Morgan, brought up a good point about tricuspid replacement and tricuspid repair during Edwards' third-quarter earnings call in October.
"This is a market we hear from doctors a lot. They may not be terribly excited about repair, but replacement could be a really exciting option," Marcus said, according to SeekingAlpha transcripts of the call. "What do you think Edwards needs to do as a company to take this market and accelerate adoption and drive uptake here in an area that's been pretty slow with tricuspid repair?"
CEO Bernard Zovighian said Edwards would do what it has done in TAVR for the past 15 years. That means first focusing on patient outcomes by making sure the sales team is well trained and well equipped to support the physicians. The company also intends to continue to generate more evidence to support the technology.
"As Bernard has said, we really do believe that it's the comprehensive high-touch model and outstanding outcomes is a key step," Chopra added. "But it's much more than that. And us having the ability to take the TAVR playbook from long ago and help apply it to this new area is really important."
That includes not only the technology, innovation, and evidence, but helping to improve diagnosis referral and, eventually, treatment of patients, Chopra said.
"But for us, this is a comprehensive kind of play, a long-term play for us to really build a new therapy that we're really excited about to help patients with," he said.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
