No Oops About It, Edwards Did It Again
The company scored the world’s first regulatory win for a transcatheter tricuspid valve replacement system to treat tricuspid regurgitation.
October 20, 2023
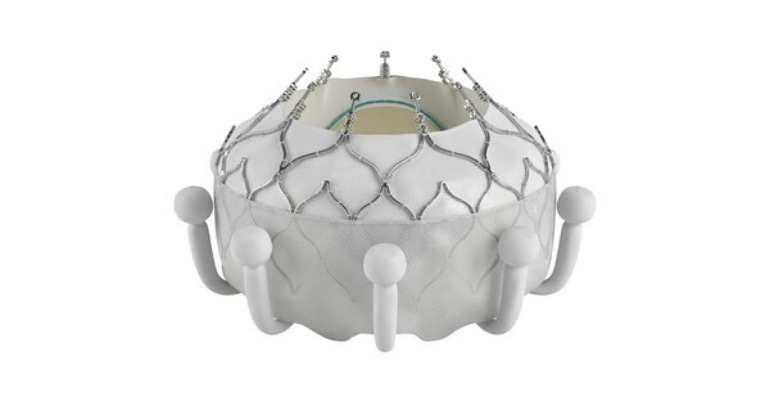
Just a smidge over 16 years ago, Edwards Lifesciences made heart valve history when it brought the first transcatheter aortic valve replacement (TAVR) system to market in Europe. Now, the TAVR pioneer is leading the way again – this time in the transcatheter tricuspid valve replacement (TTVR) market.
The Irvine, CA-based company received a CE mark for its Evoque TTVR system for the treatment of eligible patients with tricuspid regurgitation (TR). The Evoque system is the world's first transcatheter valve replacement therapy to receive regulatory approval to treat TR. This time around, however, there shouldn't be a four-year lag before FDA approval, as was the case with the company's Sapien TAVR system.
"Innovating for unmet patient needs is at the center of everything we do at Edwards, which makes us especially proud to have received CE mark for this first-of-its-kind transcatheter tricuspid valve replacement therapy," said Daveen Chopra, Edwards' corporate vice president of transcatheter mitral and tricuspid therapies. "With the Evoque system's approval, in addition to our current Pascal tricuspid system, we are now able to provide a broader array of much-needed treatment options for appropriate tricuspid disease patients in Europe."
The Evoque TTVR system is comprised of a nitinol self-expanding frame, intra-annular sealing skirt, and tissue leaflets made from bovine pericardial tissue. The Evoque valve will be available in three sizes, all delivered through a low-profile transfemoral 28 French system.
During the company's second-quarter earnings call in July, Edwards Lifesciences CEO Bernard Zovighian, said the Evoque system is on track for FDA approval around the end of 2024.
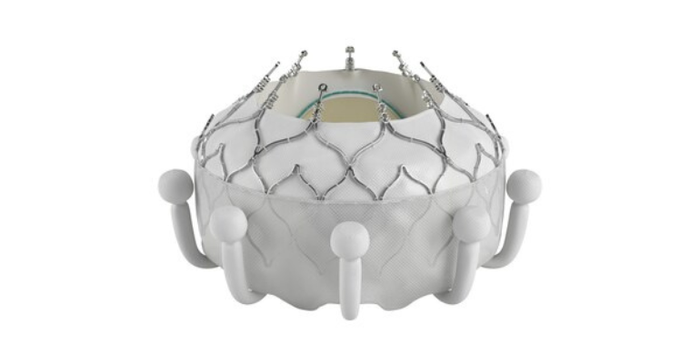
One-year results on patients treated in the single-arm, prospective, global, multi-center TRISCEND study of the Evoque system were presented at PCR London Valves 2022 and demonstrated favorable safety and effectiveness outcomes and significant quality-of-life improvements. Key findings included high survival (90.1%) and high freedom from heart failure hospitalization (88.4%); significant and sustained TR reduction to mild or trace TR (97.6%); and significantly improved functional and quality-of-life outcomes (93% of patients in NYHA Class I or II compared to 26% at baseline and a 26-point increase in KCCQ score over baseline).
"The Evoque system is able to fully replace the tricuspid valve, virtually eliminating tricuspid regurgitation in a wide range of anatomies," said Philipp Lurz, director of cardiology at the University of Mainz in Germany and European principal investigator for the TRISCEND II study. "The significant improvements in patients' quality-of-life are remarkable, now offering a therapy to many patients who previously had no treatment options."
The company plans to present results from the TRISCEND II pivotal trial of the Evoque system on Thursday at Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation.
"Arguably, Evoque is your most important pipeline product," Larry Biegelsen, a senior medtech analyst at Wells Fargo, said during Edwards' second-quarter earnings call. "...What would success look like to you, given the TRILUMINATE results showed only a quality-of-life benefit, but no positive trends on hard outcomes?"
TRILUMINATE is a randomized controlled trial sponsored by Abbott that is designed to compare transcatheter edge-to-edge repair (TEER) with Abbott’s TriClip device to medical therapy in 700 patients with severe TR, who are at intermediate or greater estimated risk for surgery.
Zovighian struggled with Biegelsen's question, noting that TRILUMINATE evaluated a different technology, given that it's a TEER device rather than a transcatheter tricuspid valve replacement device. "So, it's very tough to comment on that, but I have to say that quality-of-life is super important for patients."
Chopra made an interesting point, however.
"For us, I'm actually pretty excited by what we've seen actually previously with TRILUMINATE. It helps confirm outcomes that have come from other studies with TEER technologies that we have on PASCAL device, that TEER in tricuspid delivers great [TR] reduction with meaningful quality-of-life improvements and, we are excited to see randomized data," Chopra said. "So, to me, these are all great positive tailwinds in tricuspid and we're excited to see what the TRISCEND II interim planned analysis will show."
Biegelsen also questioned the assumed timeline of FDA approval for the Evoque transcatheter tricuspid valve replacement system. Zovighian said the end of 2024 is a reasonable estimation because the company needs to have one-year follow-up data from TRISCEND II and then present that, along with all its other data on the technology, to FDA.
"Are we going to go faster? For sure," he said. "But I think end of 2024 is reasonable."
Edwards is accustomed to moving first
In the case of transcatheter tricuspid valve replacement, will going first be a boon or a bust? Mike Mussallem, Edwards' former CEO, once acknowledged that it can be tough to pave the way, a lesson the company learned with TAVR.
"Obviously, it sounds great to go first," Mussallem told MD+DI in 2016, after our editors named Edwards the Medtech Company of the Year. "I think we realized that there's a downside to that, which is there is no pathway, there is no road. It really needs to be established. You're heading into significant uncertainty on almost every dimension . . . It's easier to go second, behind somebody else."
On the other hand, the former CEO pointed out that the value of going first is that it puts the company in the position to learn the most and the fastest.
"We like those leading-edge conversations [with physicians and regulators]," Mussallem said. "We feel like we learn a lot from that, and it helps drive our intent."
"Our approach was to really try and learn fast and improve . . . As good as the first-generation therapies were, we knew there were opportunities to make it better," he said.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
