Ahead of J&J Merger, Abiomed Achieves Milestones
The Danvers, MA-based company made progress with the Impella ECP Pivotal Trial.
December 21, 2022
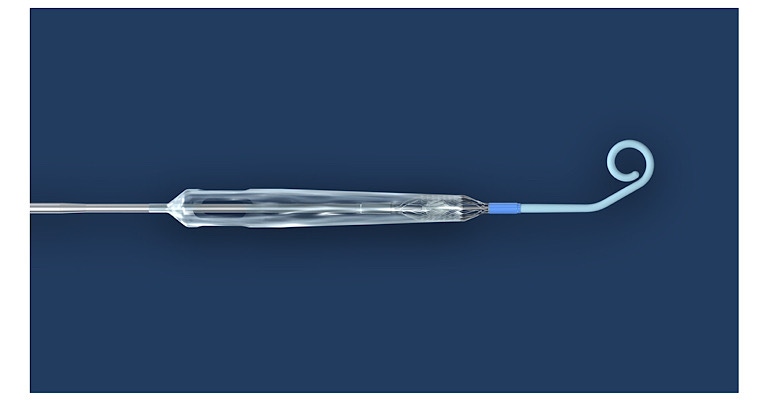
Abiomed might be on the verge of being acquired by Johnson & Johnson, but that doesn’t mean the milestones have to stop. FDA has approved the version of the Danvers, MA-based company’s heart pump that will be used in the Impella ECP Pivotal trial.
Abiomed said the first two patients have been enrolled in the trial by teams at Ascension St. John Hospital in Detroit.
The Impella ECP Pivotal Trial is a single-arm, prospective, multi-center trial that will evaluate the rate of major adverse cardiovascular and cerebrovascular events (MACCE) in adult patients who receive Impella ECP support during an elective or urgent high-risk percutaneous coronary intervention (PCI).
Both patients enrolled in the trial received Impella ECP support during challenging left main coronary bifurcation stent procedures involving heavily calcified lesions. After Impella ECP was removed, the first patient was closed with an 8 Fr closure device.
“The research and clinical teams at Ascension St. John are delighted about enrolling the first patients in the Impella ECP FDA Pivotal Trial,” said Dr. Kaki, who is national principal investigator of this study. “Impella ECP advances the opportunity for physicians to provide critical hemodynamic support during high-risk PCI procedures by delivering similar or higher flow compared to other options through a smaller vascular sheath for access. This technology has the potential to improve patient safety and cath lab throughput because of the smaller arteriotomy required for pump placement.”
Abiomed inherited the Impella technology when it acquired Impella Cardiosystems AG in 2005.
The latter half of 2022 has been a busy time for Abiomed. In September, the company said FDA approved the on-label RECOVER IV randomized controlled trial (RCT) for AMI cardiogenic shock patients.
The company also noted FDA approved and closed Impella’s prospective AMI cardiogenic shock post-approval study (PAS), RECOVER III. This study gathered real-world evidence on AMI cardiogenic shock patients treated with Impella between 2017 to 2019.
The firm also tackled healthcare disparities through its PROTECT II randomized controlled trial of the Impella pump vs. an intra-aortic balloon pump.
However, Johnson & Johnson announcing it was seeking to acquire Abiomed for $16.6 billion was perhaps the biggest news of 2022 for the heart pump-maker.
About the Author(s)
You May Also Like




