Medtronic CFO: This Is the Best Pipeline We've Ever Had
These are the products that CFO Karen Parkhill says make up Medtronic's strongest pipeline yet.
March 15, 2024
.png?width=850&auto=webp&quality=95&format=jpg&disable=upscale)
At a Glance
- Medtronic's current pipeline includes the Hugo surgical robot, the EV-ICD, pulsed field ablation technologies, and more.
- CFO Karen Parkhill emphasized the turnaround of the company's diabetes business.
Back in 2019, shortly before his retirement, former Medtronic CEO Omar Ishrak described the company's product pipeline as the strongest pipeline in Medtronic's history. Now, five years later, the company is once again boasting about its pipeline.
"This is the best pipeline we've ever had across the company," Medtronic CFO Karen Parkhill said this week at the 2024 Barclays Annual Healthcare Conference.
Read on for a breakdown of the products Parkhill highlighted.
EV-ICD (extravascular Implantable cardioverter-defibrillator)
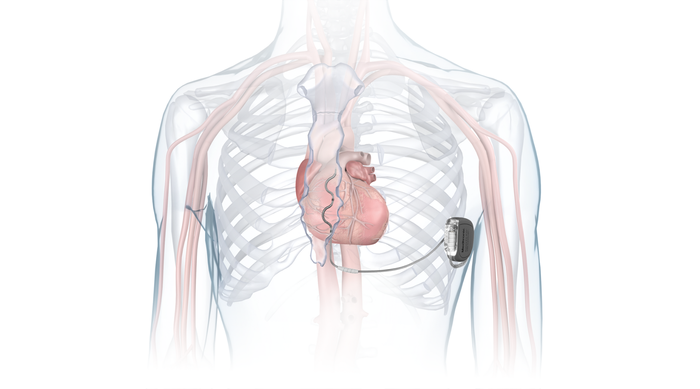
Photo illustration showing Medtronic's Aurora EV-ICD placement IMAGE COURTESY OF MEDTRONIC
Marketed as a premium product with a smaller size and extravascular positioning, distinguishing it from competitors.
Expected to be a long-term growth driver with a $300 million market already established and potential for substantial future growth.
Diabetes (780G pump, Simplera Sync sensor, next-gen innovations)
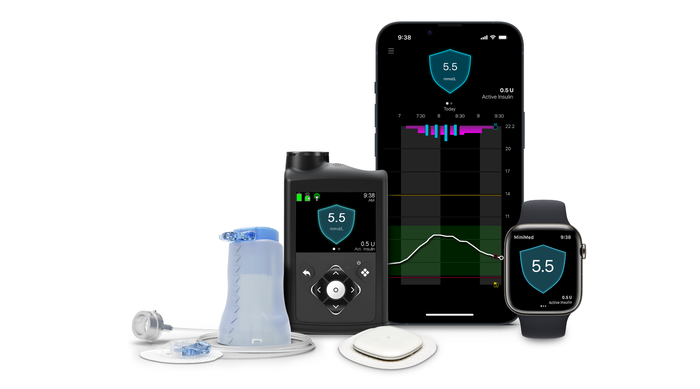
Medtronic's MiniMed 780G insulin pump system with the Simplera Sync sensor. IMAGE COURTESY OF MEDTRONIC
Significantly improved momentum in the diabetes business, with strong growth driven by the 780G pump in Europe, now expanding into the U.S. market.
Launching Simplera Sync sensor in Europe, boasting competitive advantages like smaller size, longer wear, and user-friendly features.
Continual focus on innovation with plans for next-gen algorithms, sensors, patch pumps, and a true artificial pancreas in the pipeline.
Pulsed field ablation (PFA) for atrial fibrillation (PulseSelect and Affera)
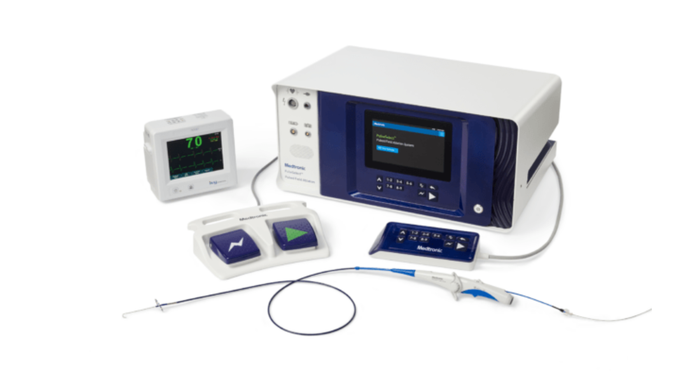
IMAGE COURTESY OF MEDTRONIC
PulseSelect, a single-shot catheter, is in limited market release, with plans for full global release.
Affera, a focal catheter with mapping technology, set to launch in Europe, expected to address 85% of the market with unique features like dual energy capabilities and all-in-one functionality.
Structural heart (Evolut FX and Evolut FX Plus)
.png?width=700&auto=webp&quality=80&disable=upscale)
Medtronic's CoreValve Evolut Pro TAVR (transcatheter aortic valve replacement) system. IMAGE COURTESY OF MEDTRONIC
Confidence in the competitive strength of Evolut FX, especially in treating small annular spaces, with anticipation for positive results from upcoming SMART trial data.
Introduction of Evolut FX Plus to address potential drawbacks of stented valves, aiming to enhance competitiveness further.
Surgical robotics (Hugo)
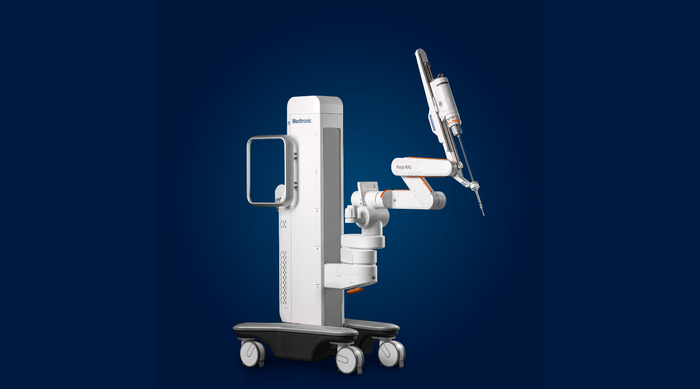
The Hugo robotic assisted surgery system from Medtronic is a modular multi-quadrant platform for soft-tissue robotic assisted surgery. IMAGE COURTESY OF MEDTRONIC
Hugo surgical robot deployed in four continents with positive feedback from surgeons and health systems on features like open console, mapping, and modular design.
In the United States, obtaining investigational device exemptions) for various surgeries including urology, gynecology, and hernia.
Go-to-market strategy includes flexible financing options to meet customer needs without competing on price, leveraging existing relationships in advanced surgery.
Medtronic is marketing both to healthcare systems and surgeons new to robotic surgery, as well as systems and surgeons that already have a da Vinci system from Intuitive Surgical.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
