Q&A.png?width=700&auto=webp&quality=80&disable=upscale)
Digital Health.png?width=700&auto=webp&quality=80&disable=upscale)
Identifying the Privacy & Ethical Pitfalls in Connected Medical DevicesIdentifying the Privacy & Ethical Pitfalls in Connected Medical Devices
Bethany Corbin details the importance of understanding these issues, citing femtech examples like reproductive health privacy post-Dobbs v. Jackson Women’s Health Organization and potential alienation of intended users.
Sign up for the QMED & MD+DI Daily newsletter.

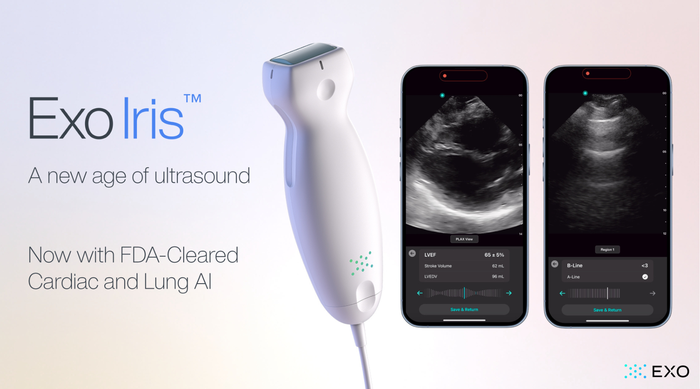



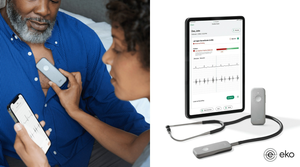





.png?width=300&auto=webp&quality=80&disable=upscale)


.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)





.png?width=300&auto=webp&quality=80&disable=upscale)




.png?width=300&auto=webp&quality=80&disable=upscale)
.png?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)



.png?width=300&auto=webp&quality=80&disable=upscale)


.jpg?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)

.png?width=300&auto=webp&quality=80&disable=upscale)
