Will HeartWare's Recall Woes Ever Stop?
June 17, 2015
The latest ventricular assist system recall involves faulty power-supply connector ports.
|
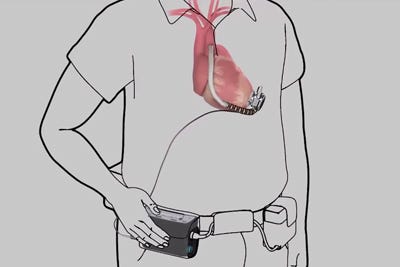
HeartWare's driveline cable, shown in this illustration from a company video, connects the implanted pump with an externally worn controller. |
Nancy Crotti
HeartWare International has recalled its ventricular assist system because the power-supply connector ports can wear down, stopping the pump and leading to patient death, according to FDA.
The Framingham, MA company has reported one serious injury and 33 complaints of malfunction. Heart-transplant candidates with end-stage, left-ventricular heart failure use the system to help deliver blood from the heart to the rest of the body, FDA said in a statement about the recall.
FDA has designated the recall as Class I.
The pump is implanted in the pericardium. An external controller manages its speed and function. The alignment guides in the power-supply connector ports may wear down over time, causing the connection pins to become twisted or bent, and eventually preventing the patient from connecting the controller to the device, FDA said.
The agency warned healthcare practitioners to consider replacing the controller if they detect damage to the connector ports.
The company issued an "urgent medical device correction" on May 11, listing the worn alignment guides among five areas of concern for patients. FDA singled out the controller issue for the recall, which covers 1,763 devices manufactured and distributed in the United States between July 2008 and March 2015.
The FDA's medical device recalls database also shows a recent serious recall involving HVAD internal controller alarm battery failures. There was also a less serious issue involving reports of discolored and cracked driveline outer sheaths.
Changes at the top of HeartWare led CEO Doug Godschall to predict the worst is behind the company, which hopes to begin a clinical trial of a miniature ventricular assist system later this year. HeartWare is working on "technical improvements, including software upgrades that will further enhance system performance and reliability," Godschall said in a first-quarter conference call on April 30.
HeartWare has had other run-ins with FDA over its heart pump, manufactured in Miami Lakes, FL.
The company received 27 complaints between February 2010 and November 2013, including reports of two deaths and four serious injuries, related to ventricular assist device system, but failed to verify or validate the effectiveness of corrective actions, according to a June 2014 FDA warning letter.
FDA also issued a Class I designation over a voluntary recall involving the system in April 2014, saying that a faulty driveline connector could cause the heart pump to temporarily stop.
Refresh your medical device industry knowledge at MEDevice San Diego, September 1-2, 2015. |
Nancy Crotti is a contributor to Qmed and MPMN.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like