Why Medtronic Has a Major Tracheostomy Tube Recall
July 15, 2015
A potentially deadly device design-related complication has Medtronic asking health providers to return thousands of tracheostomy tubes for infants and children.
|
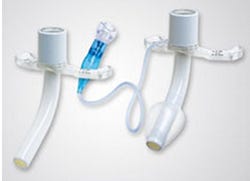
Affected Shiley tracheostomy tubes, as shown on FDA's website |
Chris Newmarker
FDA recently designated a Medtronic recall of Shiley neonatal, pediatric and long pediatric tracheostomy tubes as Class I.
The recall involves 8192 units in the U.S., with a total 69,461 units distributed worldwide, according to FDA.
It involves a device design problem.
Newer model tubes have a potentially different angle than older tubes. This can cause airway obstruction because people's windpipes can become accustomed to such a tube, which is inserted through a surgical opening through the neck and windpipe to provide an airway and to remove secretions from the lungs. Insert a newer tube with a different angle, and life-threatening problems could result.
Medtronic has reported 12 cases of serious injuries to FDA. No deaths have been reported.
Medtronic notified customers via letter on May 8, alerting them to the need to discontinue using the tracheostomy tubes and return them.
Affected tracheostomy tubes were made and distributed between May 2, 2013 to April 27, 2015. A full list of affected product and lot numbers is on FDA's website.
This is Medtronic's second Class I recall of 2015. The previous serious recall involved the Trellis 6 and Trellis 8 peripheral infusion systems.
The Trellis systems--made by what was Covidien before Medtronic closed on its $48 billion acquisition last month--have two balloons that are inflated to isolate a clot in an arm, leg, hand, or foot. Medication is released between the balloons to shrink or dissolve the clot so it can be removed. FDA blamed a "manufacturing error" for the mislabeling of the ports.
Refresh your medical device industry knowledge at MEDevice San Diego, September 1-2, 2015. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like