Standardization in Quality Management Systems: Part II
By Tim Lozier, manager for marketing and strategy at EtQ
October 16, 2011
 In the first part of this blog post, I compared multi-divisional enterprises to my two little girls, who seem to be complete opposites.
In the first part of this blog post, I compared multi-divisional enterprises to my two little girls, who seem to be complete opposites.
Let’s talk more about what is required to start a standardized process. At the core, you need to identify the processes that can give you the “early wins”: those processes that are easiest to implement and can be used as the model for future processes. You need to assemble a corporate standardization team: a group of individuals who are your best of the best. Executive-level support should drive the initiative. Standardization needs leadership to be successful, and it needs to be a priority for your organization. Some key concepts to consider when putting together a standardization project:
Simplicity: Keep it simple: complexity only drives more confusion and will lead to difficulty in user adoption. Find the most simple process path and begin with that, so you can easily incorporate it globally in the organization.
Integration: Identify the integration points and plan out where these integration points lie. Integration can be with other quality processes, other business systems or both. The important thing is that the integration makes sense when needed. An unnecessary integration or complex integrations will entice confusion (and perhaps tantrums).
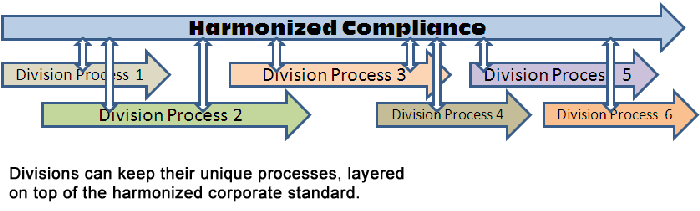
Standardized for all divisions: Important to standardization is that every division is represented in the process. True standardization will account for all divisions, and provide a standard that is acceptable across the enterprise. Some divisions may want to add more to remain unique, and that’s OK. But the key is to ensure that the framework is common (as I wrote earlier when describing how to add unique “flavor” to the standardize process).
Scalable: The beauty of a standardized process is that it is easily scaled. You can take the quality processes and easily add them to new divisions, business units, and the like without any significant changes. Even better, you can have employees switch between divisions and not have to be re-trained: the corporate standard is the only standard.
Compliant to all applicable regulations: Regulatory oversight and GMP compliance practices dictate how some processes must be executed. Important to standardization is that all regulations must be considered when standardizing the process. Some divisions may adhere to different regulations, but it is important that all regulations are represented. This will help to not only maintain the level of compliance expected, but also ensure that all divisions are operating against these regulations.
Ultimately, my kids and I find the common ground to engage in playtime activities. We usually pick something that will satisfy them both, provide the best use of our time, and hopefully tire them out so they nap for the rest of the day. Corporations seeking standardization of their quality management system need to take into account all the various divisions, and find the simplest, most logical process that satisfies each division, while ensuring the visibility and regulatory compliance that corporate is looking for.
And if that isn’t helping, maybe you need to institute a naptime program of your own.
MD+DI also referenced Lozier's writing in a recent blog post.
To read more content from Lozier, check out the EtQ blog.
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
