Standardization in Quality Management Systems: Part I
By Tim Lozier, ETQ
October 13, 2011
By Tim Lozier, manager for marketing and strategy at EtQ
 My two girls are complete opposites. One is a blonde and takes risks; the other is a brunette and is risk averse. One is a nature-freak; the other is a princess. So you can imagine that when family activities come up, each one has a different agenda. This sometimes provides a difficult task. How can we find a common activity that will incorporate frogs and lizards, but have a princess theme wrapped around it? (I won’t tell you the story of the frog that got painted pink).
My two girls are complete opposites. One is a blonde and takes risks; the other is a brunette and is risk averse. One is a nature-freak; the other is a princess. So you can imagine that when family activities come up, each one has a different agenda. This sometimes provides a difficult task. How can we find a common activity that will incorporate frogs and lizards, but have a princess theme wrapped around it? (I won’t tell you the story of the frog that got painted pink).
In many ways, multi-divisional enterprises are like my little girls. Each division is unique, with their own business processes and operating capabilities. They have a set way of doing things; so when corporate says they want to standardize quality, you can imagine the pushback (“pushback” being the professional word for “tantrum”). Yet, in many enterprises, standardization of quality management systems is not only a growing topic, it’s becoming a reality. So why standardize?
It provides a single framework for quality: With all operating companies and divisions on a single framework, it is much more manageable, consistent, and easier to identify trends.
It incorporates the best practices into common quality processes: Standardization is like the ultimate mix tape: it takes all the best practices from the organization and combines them into a single, well-defined process. The best processes are now in one standard corporate process.
It enhances synergies between divisions: If we are all operating in the same process, then we have something in common. We want the organization to operate closer. The closer we are, the better we work as an enterprise team. Standardization is the foundation for the common Quality environment.
Regulatory is raising the bar for compliance: With increased regulatory oversight comes the need to gain tighter control on the process we govern. By having a single, common standardized process, we can ensure that all operating companies or divisions are using the same process we have deemed regulatory compliant.
These are the core reasons why standardization of quality management systems is necessary in global organizations. Yet, we still have the pushback from divisional sites. The question then becomes, “how can we all be ‘common’ on a standardized platform, while being able to keep our unique site-level processes in place?” This is not an easy task, and many organizations struggle with the dynamic of creating a corporate standard, while allowing site-level processes to remain unique.
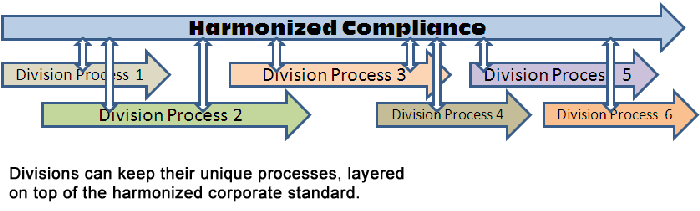
Technology has advanced over the past few years that have enabled software to segregate site-level processes from standard processes. So essentially, the corporate standard process framework stays in place, and individual sites are able to layer on their own unique processes on top. It’s like adding after-market parts to your production car: a spoiler here, a wood-grain interior there... The core engine and overall structure of the car stays the same, yet everyone can add their own unique piece to it.
This blog post is the first of a two-part series. The second part is now available.
MD+DI also referenced Lozier's writing in a recent blog post.
To read more content from Lozier, check out the EtQ blog.
Image from Flickr user Horia Varian.
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
