February 19, 2021
Peter Thor, Matt Jolly, Jordan Montgomery, Sam Owings, Sydney Heimer, Shawn Kelley, Terri Bartlett, and Molly Magnuson, Medtronic
The intent of an accelerated aging protocol is to predict and subsequently verify real-time aging behavior of a product and packaging system. A robust study is built on the foundation of prerequisite knowledge of both the use conditions of product storage and the challenges those conditions may present to the product and its packaging. Specific aspects of the product storage conditions (e.g., exposure to light, temperature, humidity) can result in degradation mechanisms of polymers, such as hydrolysis or oxidation. Understanding the degradation mechanisms of materials chosen by a manufacturer is not a simple undertaking, and companies are relying on published literature, legacy field performance data, and material specifications to make good decisions. Understanding the storage use condition is an equally daunting challenge, particularly as manufacturers are seeking to distribute products globally into all climate zones. To reflect this use condition suitably and efficiently, manufacturers are relying on the latest industry standards to guide them on how to set up and execute these aging studies. Currently, the guidance in the industry standards as it relates to using humidity in accelerated aging can lead to a variety of approaches that, while compliant, can result in accelerated aging protocols that periodically fail to successfully predict real-time aging behavior. This could be due to selection of accelerated aging conditions that are too harsh or not harsh enough, failure to understand use conditions, or other effects unintentionally caused by accelerated aging.
Humidity is a real use condition, yet surprisingly it is not always considered in accelerated aging protocols or commonly used standards for shelf aging. Over time, humidity can affect polymers in a variety of ways. Notably it can result in a relatively common polymer chemical degradation reaction known as hydrolysis, which can cause irreversible loss of mechanical properties over time. Ambient humidity levels (or the absence of humidity) during elevated-temperature accelerated aging can cause polymers to dry out to levels beyond those that would occur under ambient storage conditions. This test condition may prevent hydrolysis degradation from occurring, resulting in failure to capture degradation that would occur during real-time aging of the product (during shelf aging or use life). Further, low humidity levels at elevated temperatures can significantly alter mechanical properties, (e.g., stiffening and embrittlement of polyamides such as Nylon), which can lead to artificially accelerated failure mechanisms that may not occur during real-time aging.
Even with knowledge of material properties, unforeseen degradation from humidity can occur that might not have done so for the bulk materials alone (e.g., polymers in contact with additives like radiopacifiers or colorants), which could catalyze bulk degradation (e.g., hydrolysis). Failure to consider humidity in accelerated aging protocols can ultimately result in inadequately predicting real-time aging behavior, whether that would be missing failures that occur in the field or artificially inducing failures during accelerated aging that do not occur in the field, both of which can have significant consequences.
A potential barrier for the inclusion of humidity in accelerated aging protocols may arise from confusion around determining the appropriate levels of humidity to use, for example whether to use absolute or relative humidity and at what levels. Aging damage for many materials may be exacerbated in the presence of high or low relative humidity levels. Therefore, care should be exercised in using humidity levels that, when combined with temperature, produce moisture levels that may not be representative of expected storage moisture conditions and may cause unnatural physical changes to materials (for example, delamination of water-based laminates and coextrusions). These effects will be material-specific; for example, hygroscopic materials like polyamide-based plastics (Nylon, PEBAX, etc.); UV-cured acrylated-urethane adhesives, etc., will be affected more at elevated temperature by humidity levels than hydrophobic materials like polyethylene and silicone. Typically these physical changes occur relatively quickly (e.g., softening from absorbed water) and are usually reversible over time upon returning to ambient temperatures and humidity levels. However, they can also result in non-reversible effects such as adhesive delamination, which may not occur during real-time aging but can be artificially induced by the combination of elevated temperature and humidity levels.
Another impediment to the inclusion of humidity could be significantly increased costs required for upgrading accelerated aging capabilities, for example replacing frequently used ovens with environmental chambers. Further, this may seem burdensome or unnecessary as the majority of medical devices may not be affected by humidity.
To better answer the question of whether to use absolute or relative humidity in accelerated aging protocols, it is important to understand the relationships that govern the rate of polymer degradation and polymer mechanical properties. The theory section will first introduce polymer chemical degradation, specifically hydrolysis, where water can cleave susceptible polymer chains, resulting in molecular weight loss and loss of mechanical properties.
Understanding Polymer Degradation
To understand the equations that govern accelerated aging, it is important to first understand the chemical kinetics. Consider a chemical equation of the form:
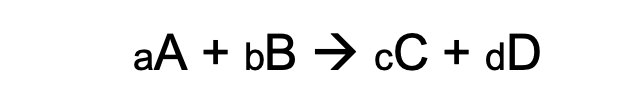
This chemical equation follows the universal rate law:
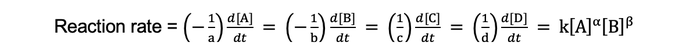
where A, B, C, and D are unique chemical species, a, b, c, and d are stoichiometric coefficients, α and β are reaction rate orders, and k is a reaction rate constant that obeys the Arrhenius relationship. Note that the negative signs in the above equation are due to species A and B being consumed in the reaction, whereas as species C and D are produced.
In the case of hydrolysis due to ambient humidity in product storage, consider species A to be the polymer matrix, species B to be water, and species C and D to be degradation products of the polymer:

Thus, the rate of a hydrolysis reaction is given by the following expression:

From the equation above, the reaction rate is dependent upon three factors:
The rate constant, k: The Arrhenius relationship describes the value of k and explains how it changes as a function of temperature.
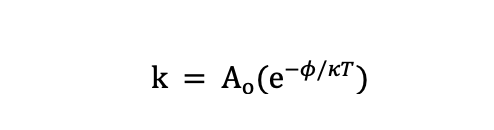
Ao = the constant for the material (frequency factor), φ is the apparent activation energy (eV); κ =Boltzmann’s constant (0.8617 X 10-4 eV/K), and T = absolute temperature (K). This equation appears nearly identically in a 1998 MD+DI article published by Hemmerich entitled “General Aging Theory and Simplified Protocol for Accelerated Aging of Medical Devices.”1 The Hemmerich paper asserts that Ao(e-ϕ/κT) is equal to the reaction rate, rather than to the rate constant, k. The underlying assumption in said article is that when accelerated aging is conducted and temperature is elevated, the other two factors, the polymer susceptible bonds and the concentration of water, do not change with temperature. This is typically a good assumption, but this article will challenge the legitimacy for various humidity conditions. The Hemmerich article has been referenced extensively for overviewing the mechanisms and equations that govern accelerated aging. Notably, Figure 1 from the paper is printed in ASTM F1980 and provides guidance around how to use the “Q10=2” guideline for accelerated aging. The “Q10=2” rule is derived from the Arrhenius relationship. It calculates the reaction rate at two different temperatures to find an acceleration factor (i.e., the ratio of the two reaction rates). This is a good approximation as long as the assumptions listed in the Hemmerich article are met (e.g., the apparent activation energy φ must be close to 0.7 eV and no thermal transitions can be crossed). Additionally, the concentration of species A and B must remain relatively constant when temperature is increased.
The concentration of polymer susceptible bonds for hydrolysis (reactant A): Testing for molecular weight changes can monitor the extent of the reaction in a polymer. However, the desired output of accelerated aging is the effect on the material with elevated temperature, so the test method by definition cannot alter the material to change the accelerated aging conditions. Further, for low extents of reaction, this can be considered a constant. This is because if the number average molecular weight is cut in half (extreme degradation by most shelf aging standards), a polymer that is 1000 repeat units long, for example, will only experience a 0.1% reduction in susceptible bonds (i.e., an average of one bond is broken per chain). Thus, the assumption that this term remains constant is very good, especially compared to the assumptions built in to the “Q10=2” guideline.
The concentration of water in the polymer matrix (reactant B): Intuitively, as more water is present in the polymer, the faster the hydrolysis will be. Ultimately, to be able to use the “Q10=2” guideline for accelerated aging hydrolysis mechanisms due to ambient humidity, it is critical that the concentration of water in the polymer is equivalent at room temperature and elevated temperature. Gardner and Martin2 note that the hydrolysis is dependent upon both the temperature and the concentration of the reactants, noting specifically that the concentration of water absorbed in the polymer dictates the rate of the reaction.
For the case of oxygen-related mechanisms, where reactant B is O2 or some sort of reactive oxygen species proportional to O2 in the air, or in the case of degradation mechanisms that require only the materials interacting with each other, the assumption that the concentration of species B remains constant at different temperatures is typically a good assumption. Hydrolysis due to ambient humidity does not fit this assumption because humidity and equilibrium moisture water content of a polymer are temperature-dependent phenomena.
Because the Hemmerich article provides a sound theoretical basis for accelerated aging reactions using the Arrhenius relationship, this paper will focus primarily on humidity as a use condition for accelerated aging (bullet 3). The absorbed moisture content in polymers is important regarding physical and chemical behavior. For many materials, aging damage may be exacerbated in the presence of high or low relative humidity levels. For example, aging at a relative humidity level that is too low may result in not observing hydrolysis degradation, which could occur under ambient use conditions. Aging at too high relative humidity levels, particularly at elevated temperatures, may cause unnatural and irrelevant physical changes (e.g., softening, adhesive delamination, swelling, densification) that do not occur under ambient use conditions. The use conditions under which a product can be stored and the failure mechanisms those conditions can induce serve as fundamental inputs to selecting temperatures and humidity to use during accelerated aging. The ultimate goal of accelerated shelf life aging is to more quickly induce only the failure mechanisms a material or device could experience as a result of real-time shelf aging.
Relative Humidity or Absolute Humidity?
The two most common practices for the inclusion of humidity in accelerated aging protocols are to 1) maintain constant relative humidity as temperature is increased or to 2) maintain constant absolute humidity as temperature is increased. For example, ASTM F1980-16 Table X33 provides guidance around equivalent absolute humidity content across temperature domains.
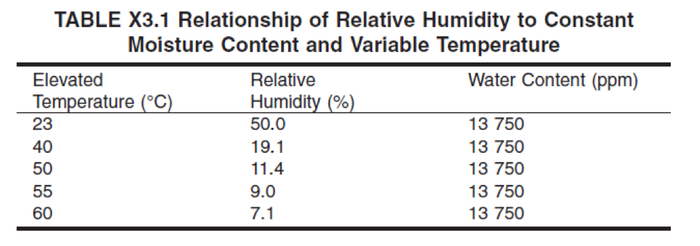
Table I. Table X3.1 from ASTM F1980-16. Reprinted, with permission, from ASTM F1980-16 Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices, copyright ASTM International,100 Barr Harbor Drive, West Conshohocken, PA 19428. A copy of the complete standard may be obtained from ASTM International, www.astm.org."
The absolute humidity is the absolute pressure that water exerts in air. In Table 1, the absolute humidity is expressed as “water content” in units of ppm. This is calculated by normalizing the absolute humidity (units of pressure) to the pressure of the ambient air and multiplied by 106 to achieve units of ppm. The numbers calculated for water content are approximately valid at sea level (pressure close to 1 atm) but will change at different ambient air pressures.
Relative humidity is the absolute humidity (units of pressure) normalized to the saturated vapor pressure of water at that temperature. As the temperature increases, the saturated vapor pressure increases (i.e., the amount of pressure that liquid water exerts in the air). Thus, if air temperature increases, and no new water is added to the system, the relative humidity will decrease as the absolute humidity remains constant as shown in Table 1. This situation is more typical of thermal aging in ovens without humidity control or low relative humidity. According to Table 1, should constant absolute humidity be selected at 55oC to represent real-time 23oC aging at 50% RH, the oven chamber would only be at 9% relative humidity.
There has not been a consensus in accelerated aging theory as to maintain constant relative humidity or constant absolute humidity when increasing temperature for accelerated aging. In order to answer this question, it is important to test the absorbed moisture content of various polymers at these humidity conditions: 23oC and 50% RH (ambient), 55oC and 50% RH (accelerated aging conditions with constant relative humidity), 55oC and 10% RH (accelerated aging conditions with constant absolute humidity).
As shown in Figure 1 below, the results overwhelmingly indicate that water absorption appears proportional to relative humidity rather than absolute humidity for two different hygroscopic polymers when temperature was increased to 55 oC from room temperature. It is worth noting that the inclusion of humidity is pertinent only for polymers that absorb or otherwise interact with water because their physical and chemical properties can be affected.
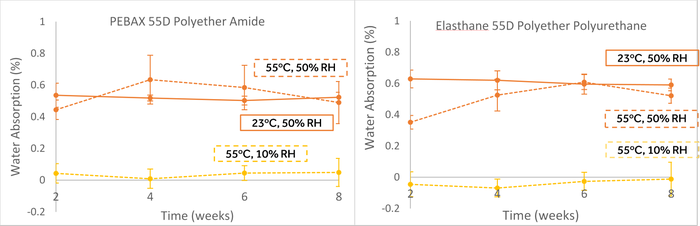
Figure 1. Moisture content of two different hygroscopic polymers (n=10, 95% confidence bounds shown) demonstrates moisture content of the polymer is dependent upon relative humidity and that using absolute humidity will result in drying out the polymer (shown by the yellow dashed lines, at bottom).
Aging polymers with constant absolute humidity resulted in drying the polymers out, whereas maintaining 50% relative humidity at elevated temperature resulted in a more similar absorbed moisture content when compared to 23°C, 50% RH. This may seem like an unintuitive result. However, there are inter-dependent physical relationships relating the temperature-dependence of vapor pressure and the equilibrium moisture content of polymers (partition coefficient), which reconcile these findings that are not covered here. Briefly summarized, the absolute humidity increases with increased temperature at a constant relative humidity, but the moisture content of the polymer is offset by a proportional decrease in the partition coefficient of water absorption of polymers. The absorbed moisture content of the polymer is dependent upon the product of the absolute humidity and the partition coefficient of the polymer; thus, the relative humidity appears to be a good metric for maintaining approximately constant equilibrium moisture content across temperature ranges (without crossing thermal transitions).
This finding corroborates those found in other industries. The paper industry4 concludes that relative humidity is important for absorbed moisture content and that absolute humidity, while important for various calculations, has little bearing on the absorbed moisture content of paper. In the agriculture industry5, it was determined that the moisture content of five different crops was close to constant across temperatures when using relative humidity rather than absolute humidity.
This constant absorbed moisture content over prolonged periods of time (26 weeks) of samples aged with constant relative humidity resulted in reductions of number average molecular weight and ultimate tensile strength for the polyether block amide material compared to the ambient conditions (Figure 2). Note that accelerated aging the material at 10% RH yielded modest changes in the number average molecular weight and in the ultimate tensile strength. This is because the water concentration of the polymer was artificially low and the hydrolysis mechanism was proportionally slowed. This likely can explain why, when using equivalent absolute humidity for accelerated aging, it is common to miss hydrolysis failure modes.
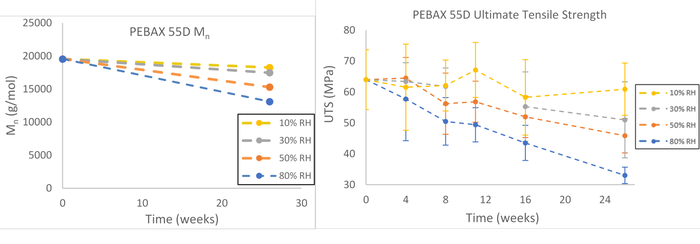
Figure 2. Number average molecular weight (left) and ultimate tensile strength (right) of PEBAX 55D up to 26 weeks exposure to various humidity levels at 55oC indicates the degradation rate is dependent upon absorbed moisture content, meaning that the degradation reaction rate (via hydrolysis) approaches 0 as the relative humidity decreases.
Implications
While using relative humidity for accelerated aging is important for revealing hydrolysis degradation mechanisms, there are important physical consequences of using inappropriate humidity levels during accelerated aging. When using humidity levels that are too low, materials like polyamides (e.g., Nylon) can become unnaturally stiff and brittle, resulting in a change in device or packaging performance. This physical embrittlement is a reversible phenomenon in that the mechanical properties of the material will return over time when exposed to nominal humidity levels. However, brittle device failures and packaging delamination can occur while aged under extremely dry conditions, which are irreversible consequences of selecting poor accelerated aging conditions. This physical embrittlement of Nylon has been researched and documented for medical applications like catheters.6
Conversely, another common example of physical consequences of incorrect accelerated aging conditions is the unnatural softening of materials when exposed to high humidity in combination with high temperature. For example, Dubelley7 et al discovered the time-dependent water absorption of amorphous polyethylene terephthalate deviated from traditional Fickian diffusion above 50% RH and 50oC. This unnatural absorption of water at high temperature and high humidity can induce unnatural physical changes in hygroscopic polymers that otherwise would not occur in nature. These changes can occur when the polymers are exposed to temperature and relative humidity conditions that could never occur in nature, causing unnatural water absorption levels that result in softening, delamination, etc., which do not occur under real ambient aging conditions. Ultimately the goal of accelerated aging is to simulate use conditions that are conducive to real changes in polymer behavior yet avoid significant unnatural phenomena (e.g., plasticization, delamination, softening, etc.) As such, caution should be exercised when conducting accelerated aging with humidity levels above 55% RH at temperatures above 55 oC for prolonged periods of time.
Figure 3 below shows the effects of accelerated aging a typical UV-cured acrylated urethane adhesive. This adhesive has years of use in the field and has demonstrated robust performance when aged at room temperature. Accelerated aging was conducted at three different conditions: 1) 50oC and 65% RH (orange), 2) 60oC and 65% RH (grey), and 3) 65oC and 90% RH (blue).
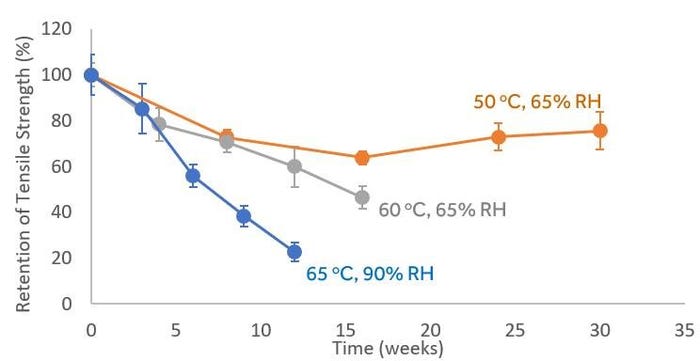
Figure 3. Retention of tensile strength of a typical UV-cured acrylated urethane adhesive (n=5, 95% confidence interval, etc.) with aging at elevated temperature and humidity.
The combination of high temperature and high humidity appeared to result in the unnatural plasticization of the adhesive, which caused the unnatural reduction in mechanical properties. When accelerated aging was conducted at 60oC and 65 RH and at 65 oC and 90% RH, the ultimate tensile strength of the adhesive continually dropped over time. However, at a more modest 50oC, 65% RH, the material more closely matched the real-time observation of persistent mechanical properties. It is worth noting that this particular class of adhesives is very hygroscopic and that water absorption levels up to 6% w/w were observed. This type of behavior would not be expected for polymers that are hydrophobic (e.g., polyolefins, silicones, etc.), which typically absorb water at <0.5% w/w. The minimum condition that causes unnatural reduction in mechanical properties is a function of many factors: temperature, humidity, material, time, etc. However, our data with a more ‘worse’-case humidity-sensitive material provides confidence that recommended aging conditions (e.g., 55oC and 55% RH) should not result in unnatural phenomenon (e.g., softening).
Importance of Humidity Control
A practical consequence of not controlling humidity (e.g., using typical ovens without humidity control) will result in aging similar to using absolute humidity, but it will depend slightly on the ambient humidity of the room. The reason is because ovens take ambient air, heat it up, and force it through the chamber. The air that the oven takes from the ambient room will have some amount of humidity present (e.g., 50% RH at 23 oC = 13750 ppm at sea level). When this air is heated in the oven, the temperature increases, but the absolute humidity will remain constant because no new water is added or removed from the air. This results in air with the same absolute humidity, but at a different temperature (e.g., 13750 ppm = 9% RH at 55 oC at sea level). This will vary depending on the ambient air. For example, labs in the winter of Minnesota may read 12-15% RH because the air has already been heated from -20 oC outside, and reheating it will dry it out further. During the summertime, the labs may be at ~50% RH. Regardless, this situation will more closely mimic the fixed absolute humidity condition than the constant relative humidity, with slight differences caused by fluctuations in the inlet humidity/temperature and, of course, the set point temperature of the aging.
Ultimately, it is recommended to use relative humidity in modest levels (e.g., ~45-55% RH) for accelerated aging of polymers. Too high of relative humidity can result in unnatural physical changes, and too low of humidity will result in unnaturally drying out the polymer. For polymers that do not absorb moisture, inclusion of relative humidity in accelerated aging may not be critical (e.g., polyolefins, polystyrene, PTFE). However, for hygroscopic polymers, particularly those compounded with additives or those that contact other materials directly (e.g., adhesives), it is highly recommended to consider aging with a modest level of relative humidity.
Below is a list of common hygroscopic and/or hydrolytically susceptible polymer classes:
Polyamides (Nylons, etc.)
Polyesters
Polyurethanes
Polyacrylates (e.g., cyanoacrylate adhesives)
Polycarbonates
Plasticized polyvinyl chloride (PVC)
Polyvinyl alcohol (PVA)
Cellulose
Polyethylene glycol-based coatings (PEG)
Polyvinylpyrrolidone (PVP)
Resorbable polymers (e.g., Polylactide {PLA}, Polyglycolides {PGA}, etc.)
References
Hemmerich, KJ. “General Aging Theory and Simplified Protocol for Accelerated Aging of Medical Devices.” Med Dev Diag Indus, 1998. https://www.mddionline.com/design-engineering/general-aging-theory-and-simplified-protocol-accelerated-aging-medical-devices
Gardner, RJ and Martin, JR. “Humid aging of plastics: Effect of molecular weight on mechanical properties and fracture morphology of polycarbonate.” Journal of Applied Polymer Science, 1979. https://onlinelibrary.wiley.com/doi/abs/10.1002/app.1979.070240512
“ASTM F1980-16, Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices.” ASTM International, West Conshohocken, PA, 2016. http://www.astm.org/cgi-bin/resolver.cgi?F1980
High, L. “Paper Moisture and Relative Humidity.” Glatfelter’s Technical bulleting, Issue 2005-2, 2005. https://pdfs.semanticscholar.org/b4fb/b2552971ca4639ec93c1a6bb1a2c7c8d8e0c.pdf
Sadaka, S. and Bautista, R. “Grain Drying Tools: Equilibrium Moisture Content Tables and Psychrometric Charts.” Univeristy of Arkansas Division of Agriculture. https://www.uaex.edu/publications/pdf/FSA-1074.pdf
Touris, A, et al. “Effect of Molecular Weight and Hydration on the Tensile Properties of Polyamide 12.” Results in Materials, 2020. https://www.sciencedirect.com/science/article/pii/S2590048X20300911
Dubelley, F, et al. “Water Vapor Sorption Properties of Polyethylene Terephthalate over a Wide Range of Humidity and Temperature.” Journal of Physical Chemistry B, 2017. https://pubmed.ncbi.nlm.nih.gov/28121446/
Peter Thor serves as scientist, Matthew Jolly as senior principal scientist, Jordan Montgomery as distinguished packaging engineer, Sam Owings as principal mechanical design engineer, Sydney Heimer as scientist, Shawn Kelley as distinguished scientist, Terri Bartlett as engineering technician, and Molly Magnusen as chemist I for Medtronic.
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
