How to Deal with a Medical Device Recall: An Expert Weighs In
August 28, 2013
|
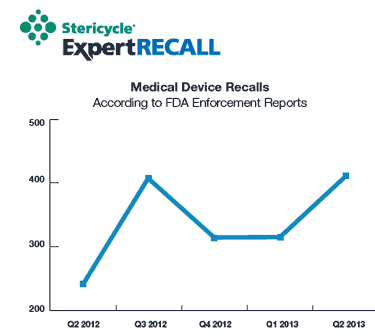
Medical device recalls ticked up in the second quarter of 2013. Image from Stericycle ExpertRECALL. |
Medical device recalls at a five-quarter high and dealing with them has become increasingly complex as a result of globalization. "The number of recalls that had a global component were up, which really speaks to the challenges of a global supply chain," says Mike Rozembajgier, vice president of recalls for Stericycle ExpertRECALL.
In the second quarter of 2013, medical device recalls ticked up 30% over the previous quarter. Nevertheless, the total number of units recalled globally dipped--by 50% in that quarter.
In addition to the number of recalls that affected units both in the U.S. and abroad, the global supply chain is complicating matters in that many medical devices contain components developed outside of the country. "How medical device companies go about minimizing the ensuing complexity goes back to their internal quality programs, their ability to conduct appropriate audits of their facilities, and having an appropriate recall in place to manage the logistics in a timely fashion," says Rozembajgier. Medical device companies need to make sure that they are transparent in the manner in which they communicate and that they have the appropriate logistical plan in place to manage the flow of product back to a central source so it can be removed from the marketplace."
Depending on the target demographic involved, medical device companies could consider using social media to help get the message out about the recall, Rozembajgier says. "If it is a device that has broad-based distribution or one that has an over-the-counter unit, they could use that route to help get message out in a format that is clear and concise." The pharmaceutical industry has used this tactic successfully, but social media has not been widely adopted for this purpose by medtech companies.
For more on recalls:
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
