Analyze This: Device Cleanliness Testing
A scientist weighs a crucible for gravimetric analysis.
Most medical device manufacturers know the importance of putting a product through rigorous cleaning and testing before it is released into the market. Standard sterility and biocompatibility tests are an essential part of manufacturing protocols. However, some manufacturers may not fully understand the different ways a product can be contaminated during the production, packaging, and cleaning processes. To avoid detrimental contamination, manufacturers should test their devices, including those made via injection molding, using residual manufacturing materials (RMM) analysis.
The Basics of RMM Analysis
Any contaminants such as oils, lubricants, releasing agents, and detergents transferred to the product during the manufacturing or cleaning process are RMM. From extracting the device out of the mold to cleaning and packaging, device contamination can occur just about anywhere along the production process. Those residues that remain on a product can be potentially cytotoxic and harmful, particularly for devices implanted inside the body. For example, a metal device may be designed to integrate with a patient’s bone, but oils remaining on the metal could reduce new bone growth or inhibit integration, causing the device to be ineffective or unsafe.
RMM analysis quantifies the residuals on the device and identifies them. It establishes a baseline against which manufacturers can discover if the amount of residuals changes as they improve their assembly process.
Manufacturers can use RMM analysis as a tool to monitor the cleanliness of production at any point, including the final product. Knowing how much residue is on a device or component allows for the establishment of effective cleaning procedures that are crucial to the release of clean and safe medical devices.
RMM analysis uses three methods to evaluate the different phases of manufacturing and cleaning—gravimetric analysis, total organic carbon (TOC) analysis, and detergent residual analysis by ultraviolet/visible (UV/VIS) spectroscopy. All three tests are quantitative and designed to remove surface contamination but are not intended to remove or assess leachable components from a device. The assessment from these tests can be used to determine cleaning efficiency as well as aid in the validations of cleaning and rinsing methods.
Gravimetric Analysis (ASTM F2459-05). Quantifying extractible residue by gravimetric analysis involves using aqueous and nonaqueous solvents to extract contaminants such as oils, salts, and other materials from the surface of medical devices.1 After the device is extracted, the solvent is evaporated and the remaining residuals are weighed and quantified. Gravimetric analysis does not determine the specific elements making up the residue, but it measures the quantity of the total amount of residue coming off the device.
If the analysis quantifies significant residue, the laboratory may also identify, or qualify, residue by Fourier transform infrared spectroscopy. A general analysis or interpretation of the sample spectrum can reveal the presence of certain types of compounds such as hydrocarbons and amines. The laboratory can also identify the compounds by comparing the sample spectrum to the spectra of target compounds. Identifying the residue may be important when a manufacturer is trying to control the source of residue production.
TOC Analysis. TOC analysis is the most sensitive of the RMM series of tests. The analysis is quantitative and detects carbon-based materials such as oils, adhesives, and detergents on a product, but does not pick up inorganic residue such as metals and salts.
TOC analysis involves extracting devices in USP-purified water by sonicating or shaking, to remove surface contaminants. An aliquot of the extraction solvent is analyzed on a TOC instrument to determine how much organic carbon is on a device or component. Alternatively, the manufacturer can use swabs to evaluate clean-in-place components and equipment. After swabbing the targeted component, the swab is sent to the lab to be immersed in USP-purified water and analyzed.
The manufacturer must evaluate the results and determine how much residual material is allowable based on the device’s designed use. For example, if a device is designed to be implanted in the body, it needs to carry fewer organic residuals than if it is a smaller, functional part of a bigger device used outside the body, such as a gear in a machine.
Detergent Residual Analysis. Manufacturers use a variety of cleaning agents to clean their devices. Unfortunately, these cleaning agents can leave behind potentially harmful residual material. Detergent residual analysis detects detergents by UV/VIS spectroscopy.
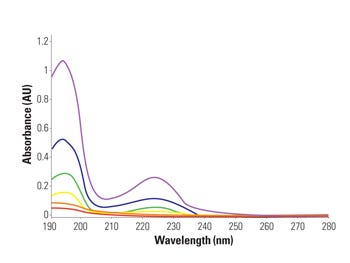
|
Figure 1. An example of a detergent residual analysis U/V printout. |
Because each detergent absorbs UV light differently, the laboratory validates each one for accuracy, precision, linearity, limit of detection, and limit of quantitation. For this validation method, manufacturers must provide a full-strength sample of detergent that is used in the cleaning process. Detergent residual analysis also uses water extraction and sonication to identify the detergent left on a device. The lab compares device extracts to the detergents calibration curve for quantification (see Figure 1).
Detergent residual analysis confirms that devices are being adequately rinsed. Manufacturers, including molded device manufacturers, should consider having this analysis method performed on each type and size of device because different devices may require different or additional rinsing to completely remove detergent residuals. For example, rinsing a smooth artificial knee is much easier than rinsing a device with grooves, pockets, lumens, or mated surfaces.
Why RMM Analysis is Essential
Several critical reasons why medical device manufacturers should perform RMM analysis include the following:
Cleanliness of device: RMM analysis helps manufacturers determine whether they are producing a clean and safe device.
Cleanliness of manufacturing process: RMM analysis can determine cleanliness throughout the manufacturing process. Without this series of tests, manufacturers may not know whether they are overcleaning their devices and molds and therefore wasting time and money on unnecessary rinses and cleaning cycles. The analysis also informs the manufacturer whether its cleaning processes are working by showing how much residue is left behind on the products.
Designing cleaning processes: Manufacturers design their cleaning processes to remove residue from a device, but cleaning agents can add additional contaminants to a device and render it unclean and possibly cytotoxic. In some cases, the cleaning process can makes the device more cytotoxic than before cleaning. RMM analysis can help manufacturers ensure that cleaning and rinsing processes are effective and producing a device that is safe and patient ready.
Increased FDA involvement: FDA is increasingly examining the cleanliness of medical devices. RMM analysis provides substantiated proof that products have undergone meticulous cleanliness testing. This documentation can be extremely useful to support a regulatory submission or in the event of an FDA or notified body audit.
Mold-release agent transfer: Manufacturers often use mold-release agents to remove devices from a mold, which can leave residue on the device. RMM analysis ensures that the device is not picking up unexpected residues or that these residuals are at acceptable levels postproduction.
Creating a baseline measurement: RMM analysis creates a baseline or a gauge for a cleaning process. This can serve as a useful validation criterion to ensure consistent production of a safe product.
|
A scientist loads samples on a TOC analyzer. |
Conclusion
There are no established regulatory limits of cleanliness for residual analysis. Manufacturers should perform a risk assessment to determine acceptable levels of residue based on the application and patient-contact duration. They should also establish limits for the cleanliness and safety of their medical products.
Medical device manufacturers can save time and money down the road if they analyze their devices, including those made in molds, for RMM. The analysis method streamlines and enhances process efficiency to ensure safety while reducing product and regulatory liabilities. Conducting such analysis can prevent manufacturing from halting when a problem or changes in the process arises.
References
1. ASTM F2459-05. 2005, “Standard Test Method for Extracting Residue from Metallic Medical Components and Quantifying via Gravimetric Analysis” (West Conshohocken, PA: ASTM International, 2005).
Tina May is chemistry section manager at Nelson Laboratories (Salt Lake City). Brent Shelley is study director at the company.
About the Author(s)
You May Also Like