Hologic Scores Regulatory Nods in EU and Canada for Endometrial Ablation Device
The company won regulatory approval in Canada and Europe for an updated version of its NovaSure endometrial ablation device.
February 15, 2023
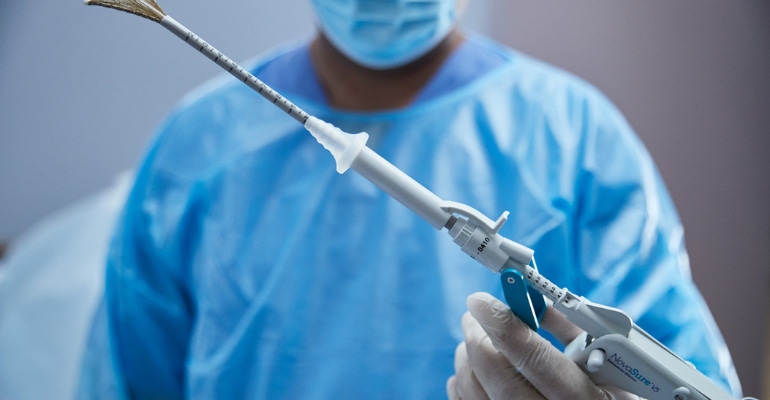
Hologic, one of the few companies to emerge from the pandemic stronger than it was before, has scored regulatory approvals in Canada and Europe for an updated version of its NovaSure V5 endometrial ablation device.
The Marlborough, MA-based company launched the NovaSure device more than 20 years ago, and customer feedback has driven continuous design improvements since then, said Jan Verstreken, group president of Hologic's international business.
“This has enabled us to develop our technology to further meet their needs and those of the women they treat, ultimately delivering better outcomes for these women," Verstreken said.
NovaSure V5 endometrial ablation device has an updated cervical seal, featuring EndoForm technology, designed to increase the sealing surface and accommodate a range of cervical canals and anatomical variability. The device’s AccuSheath markings are designed to improve the accuracy and confidence of seating and fundal placement. Additionally, the NovaSure V5 endometrial device is equipped with SureClear technology, Hologic's unique fluid removal system, which provides integrated suction through the array by constant tissue contact while simultaneously removing ablation byproducts such as vapor and fluid.
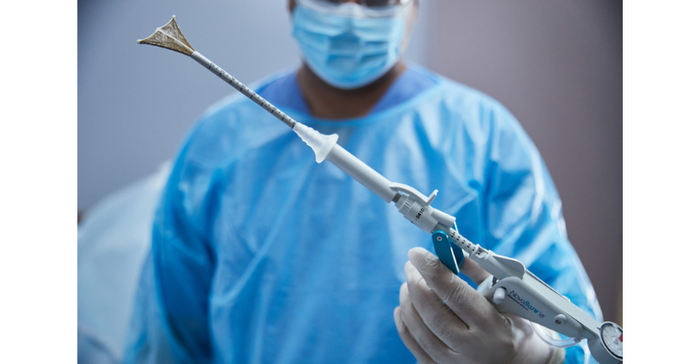
“I welcome the new features on the NovaSure V5. These will support both inexperienced and more established users in performing effective, more efficient, and accurate procedures,” said Philippe Y. Laberge, MD, a physician at the CHU de Québec-Université Laval in Québec, Canada. “The addition of markings to the shaft will enable users to correctly place the device before initiating a procedure. The cervical seal is a significant improvement as there is less chance of leaks, allowing the completion of pre-procedure checks and the procedure itself.”
According to Hologic, the NovaSure endometrial ablation device enables an endometrial ablation procedure for the treatment of abnormal uterine bleeding, with 97% patient satisfaction and 87% of patients avoiding a hysterectomy at 10 years.
Hologic's surgical business has changed dramatically over the past three years, CEO Stephen MacMillan said during the company's most recent earnings call. What once was essentially a two-product division is now much more robust and diverse. In the fiscal first quarter of 2023, revenue outside of NovaSure and MyoSure represented roughly 25% of the division's total compared to only about 10% in fiscal 2019.
"While NovaSure and core MyoSure still formed a strong durable base of the division, the business is far more dynamic than pre-pandemic," MacMillan said. "We now have meaningful growth drivers outside of these two core product lines from acquisitions, new in-house products, and also improvements to existing products."
About the Author(s)
You May Also Like




