Superbug Infections Spread by Devices
While superbug infections are nothing new, there was a surge in interest and lawsuits related to them in 2015, thanks to deadly outbreaks associated with a particular type of endoscope called a duodenoscope.
Qmed Staff
|
|
|
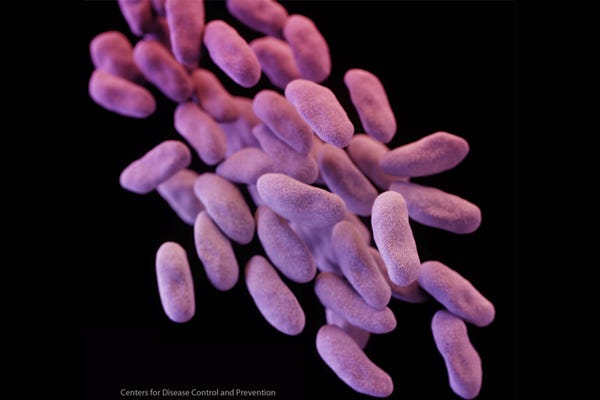
In early 2015, the Olympus TJF-Q180V duodenoscope was linked to a deadly superbug outbreak at UCLA's Ronald Reagan Medical Center in which at least seven patients were infected and two died. The hospital reported that 179 patients may have been exposed to the drug-resistant bacterial strain carbapenem-resistant Enterobacteriaceae (CRE). Another 11 CRE-related patient deaths have been linked to duodenoscope procedures at Virginia Mason Medical Center in Seattle between 2012 and 2014, and cases were also reported at Advocate Lutheran General Hospital outside Chicago.
What is especially disturbing about the story is that FDA discovered that the product in question didn't have proper regulatory clearance to be on the market. According to an FDA notice, the agency received queries from healthcare providers asking whether they should stop performing endoscopic retrograde cholangiopancreatography (ECRP)--the procedure that had been linked to the infections. FDA responded that the manufacturer had indeed filed a 510(k) application for the product, albeit belatedly, and that the agency couldn't feasibly recall the product until it received regulatory clearance because doing so would cause a product shortage for a medically-necessary procedure.
Sold in the United States by manufacturers including Fujifilm, Olympus, and Pentax, duodenoscopes provide the least invasive way of draining fluids from pancreatic and biliary ducts blocked by cancerous tumors, gallstones, or other conditions. But at this point, none of the methods proposed for cleaning the reusable devices appear to be optimal.
Duodenoscopes aren't the only devices causing worries about bacterial infections. Eight people treated with a medical device used in open-heart surgery in York, PA, ended up getting infections. Four of them died.
FDA also released warnings about Custom Ultrasonics endoscope washers and infections spread by reprocessable flexible bronchoscopes, in addition to stern warning it gave to three duodenoscope makers.
Learn more about cutting-edge medical devices at MD&M West, February 9-11 at the Anaheim Convention Center in Anaheim, CA. |
Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz. Chris Newmarker is senior editor of MPMN and Qmed. Follow him on Twitter at @newmarker
CRE image courtesy of the U.S. Centers for Disease Control.
About the Author(s)
You May Also Like