Estimation of Average Bioburden Values for Low-Bioburden Products
A key aspect of the validation of a sterilization process, irrespective of the sterilizing agent, is an understanding of the product bioburden. This understanding must be both quantitative and qualitative; it’s important to understand both the number and types of contaminating microorganisms. The challenge to a sterilization process is related to both of these measures; high numbers of contaminating microorganisms and types of microorganisms with high resistance both pose a greater challenge to any sterilization process. Validation strategies for moist heat and ethylene oxide sterilization processes require this understanding of product bioburden—it is central to the bioburden-based approach and plays a key role in the combined bioburden/biological indicator approach to process validation.
Establishing a radiation sterilization dose is based on the product bioburden’s resistance to radiation. Biological indicators are not used in the establishment of a radiation sterilization dose and their use is contraindicated. Radiation dose establishment Methods 1 and VDmax of ANSI/AAMI/ISO 11137-2 require the determination of bioburden on product items from three product batches1. An average bioburden value is determined that is either the overall average for the three batches or the highest individual batch average if it is equal to or greater than 2× the overall average value. The indicated bioburden value is carried forward in these dose establishment methods and is used in a look-up table to identify a radiation dose to carry out the verification dose experiment. Clearly, for the verification dose value to be valid, the average bioburden value must be reliably estimated2.
A Problem with Low-Bioburden Products and Radiation-Dose Establishment
Many medical products currently being developed, particularly drug/device combinations, are manufactured under highly controlled conditions, a consequence of which is very low average bioburden. This is a good thing from the point of view of challenge to the sterilization process and the extent of treatment required to attain the required sterility assurance level (SAL). The problem arises when most or all of the product items have no recoverable bioburden in spite of using an efficient bioburden determination method with a validated recovery factor.
Table 1 gives the results of bioburden testing of three batches of product in preparation for the performance of a radiation dose establishment using Method VDmax; the data are formatted in the manner that was used by the testing lab. In the method applied, each product was immersed in an extraction fluid, agitated in a prescribed manner, and one-third of the extraction fluid was filtered through each of three 0.22-µm pore size membrane filters. Two of the filters were plated individually on soybean casein digest agar; one plate was incubated aerobically, and the second anaerobically. The third filter was plated on potato dextrose agar and incubated aerobically. After the specified incubation period, the colonies on the filters were counted and the results recorded.
Table 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
No colony-forming units (CFU) were observed on any of the filters. Because only one-third of the extraction fluid was filtered onto any membrane filter, the result recorded for each plate was < 3. The problem comes with the calculation of the average bioburden—each of the < 3 entries was treated as 3, and an average of 3 was declared. As the average value was 3 for aerobes, anaerobes, and yeast/molds, the total average bioburden for each of the three batches of product was declared to be 9, and an overall average of 9 was declared for the three batches. The value of 9 was carried forward into the VDmax look-up table to determine the verification dose. It is important to reiterate that no CFU were observed on any of the plates.
The conclusion that each of the product batches had an average bioburden of 9 is neither reasonable nor statistically tenable. If the average bioburden for aerobes were in fact 3, the total number of CFU that would have been expected to be observed would be 30 on the aerobically incubated plates (the actual number of CFU expected to be recovered is 90, but only one-third of the volume was filtered, so 30 would be the observed value). A finding of no CFU for the 30 product items is not reasonable and a Poisson Distribution calculation gives a probability of ~10-13 for a finding of 0 CFU with an expected average of 30 CFU.
What is the proper estimate of the average bioburden in this situation? A value of 9 is clearly not a good estimate and the bioburden is most likely not zero either. The average bioburden is clearly < 9, but how much less than 9?
Treatment of < Values
One approach for calculating an average bioburden estimate for the case described above is based upon the Poisson Distribution. The total number of CFU observed for "aerobes" for a given product batch was zero. The 95% upper confidence limit (UCL) for an observation of zero, using the Poisson Distribution, is 3. For each product batch, with a dilution factor of 3, the calculated average bioburden estimate would be (3/10)*3 or 0.9 CFU. If this approach were applied to all of the results for aerobes, taking into account all three batches of product, the calculation would be (3/30)*3 or 0.3 CFU. This value is statistically tenable. If 0.3 were the true overall average value, again with a dilution factor of 3, the total number of expected CFU for the 30 plates would be 3 ((0.3*30)/3). The 95% lower confidence limit for an average value of 3 is zero. Another way of looking at this latter calculation is to frame it as the count found in 100% of the filtrate from 10 products rather than one-third of the filtrate from 30 products. The calculation becomes simply 3/10 or 0.3. For a single batch, filtrate equivalent to 3.33 entire products is tested so the calculation can be framed as (3/3.33) or 0.9.
This approach can be readily applied to any combination of numerical and < results. For each batch of product and each test condition (aerobic, anaerobic, yeasts/molds), add the total number of CFU observed and to this sum add the value of 3 if one or more products has no recovered bioburden. This is shown in Table 2.
Table 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
In Case 1, CFU are observed on each of the plates and the average bioburden value is accordingly the arithmetic mean of the total number of CFU observed (appropriately multiplied by any dilution or recovery factors). In Case 2, four of the plates were found to have no CFU; 3 was added to the total number of CFU observed and the calculation of the average made. Case 3 is the example in Table 1 where no CFU are observed on any on the plates. Note that if the approach used in Table 1 had been used for Case 3, an average bioburden of 3 would have been declared. This value is inconsistent with the results of Case 1 where the same average was determined based on the observation of a total of 10 CFU.
"Censored" Data Approach Substitution
Experimental data that are termed "censored" are outcomes that are below or above a limit of detection (LOD), either due to capability of the method being used or the nature of the experimental design. An analytical method might have a lower LOD of 10 parts per million (ppm). A test result where the analyte in question is not detected would be recorded as <10 ppm. The point that is not clear is how much less than 10 ppm is the true result.
An example that involves experimental design is the determination of the number of runners who finish a marathon in a time between 3 and 4 hrs. An observer could watch the entire race and have a time value recorded for all runners; in this case there would be no censored data. Or, the observer could arrive at the finish line just at the 3-hour mark and depart right at the 4-hour mark. In the first case, calculating the average finishing time for all runners in the race would be straightforward. In the second case, there would be considerable censored data. A number of runners would have their time recorded as either < 3 (“left-censored” data) or >4 hours (right-censored data).
An approach that can provide a good estimate of the average result for a data set with left-censored data is to substitute one-half of the LOD for each < value3. This substitution is most appropriate when 50% or less of the values are censored. The data shown in Table 2 were analyzed using this approach and the results are shown in Table 3. As can be seen, the result for Case 2 is similar to that shown in Table 2. The result for Case 3 is markedly higher using the one-half LOD approach. Such an outcome is expected when all of the data from a given test are censored.
Table 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Comparing the Two Approaches
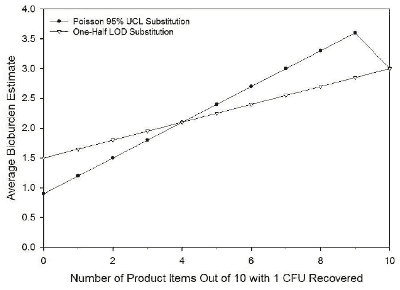
Figures 1 and 2 compare the results when the two approaches discussed above are applied. In these examples, each product has either zero or one CFU. The results cover the range from none of the products having any recovered CFU, to all of the products having 1 CFU.
As shown in Figure 1, when no or few CFU are recovered from the 10 products, the one-half LOD substitution for the censored data gives a higher estimate of the average bioburden value. As the number of products with observed CFU increases, with consequently fewer results that are “0,” the average values estimated by the two approaches converge and then diverge as the number of products with recovered CFU increases further, to a final convergence when no results are “0.”
Figure 1: Shown is a plot of the estimate of the average bioburden for a group of 10 products that each has either 0 or 1 CFU recovered, with a dilution factor of 3 and a recovery efficiency factor of 1. |
|
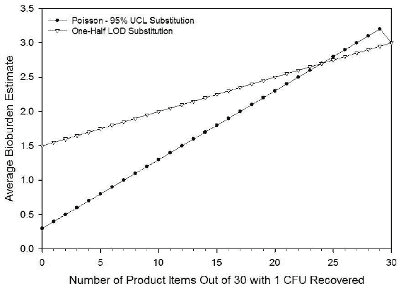
Figure 2 compares the two approaches using the results of testing 30 products as in the calculation of the overall average bioburden for three lots of product, 10 products tested per lot. The same outcome is observed as seen above, but in this case, the average bioburden estimate given by the two approaches yields the same value at a lower fraction of products with no CFU recovered. This effect is caused by the substitution, by the Poisson approach, of a value of 3 CFU which, on average calculation, is distributed over a larger number of products compared to the case illustrated in Figure 1. The one-half LOD substitution assigns the same value to each product with no recovered CFU, regardless of the number tested.
Figure 2: This plot shows the estimate of the average bioburden for a group of 30 products that each has either 0 or 1 CFU recovered with a dilution factor of 3 and a recovery efficiency factor of 1. |
|
A simple Excel spreadsheet has been developed to calculate average bioburden estimates for data sets with “<” values. Required inputs are the number of product items tested, the total number of CFU observed, the number of products with no recovered CFU, the dilution factor, and the recovery efficiency factor. The bioburden estimate is calculated by both approaches and the results compared. The lower of the two values is taken as the best estimate of the average bioburden from the inputs supplied. A screenshot of the worksheet is shown in Figure 3.
Figure 3: This calculation estimates the average bioburden using Poisson Distribution-based and one-half LOD-based substitution approaches. It shows a test of ten products, each of which has no recovered CFU, and a dilution factor and recovery efficiency of 1. | ||||||||
|
Optimizing Bioburden Recovery Methods
Clause 7.2.3.2 of ISO 11137-2 notes that for products with a low average bioburden, multiple products may be pooled and tested together in the same extraction vessel. If ten products were tested in this manner and a total of 15 CFU were recovered on the aerobic plates, a batch average bioburden of 1.5 CFU could be inferred for aerobes, assuming all of the extraction fluid was assayed and the recovery efficiency factor was 1.
The use of a pooled sample method requires that a recovery efficiency be determined for that approach and it’s important to note that there is also a loss of information with respect to bioburden distribution from item to item. In the example above, was there a low CFU level on most of the ten products, or did one product have 15 CFU and the others none?
Combining/Eliminating Tests
In too many instances, manufacturers default to a "four way" bioburden test: aerobes, anaerobes, yeasts/molds, and aerobic spores. To accomplish this test approach, the extraction fluid is divided into at least four parts which automatically results in a dilution factor of 4.
In most cases, testing for spores generally brings little added value. The presence of spore-forming microorganisms can be readily determined during simple identification testing on the bioburden recovered on the aerobic plates. If an election is made to test for spores initially, this test can be subsequently eliminated unless some particular value has been demonstrated.
Similarly, testing for anaerobes generally brings little value. Most synthetic medical products are free from strict anaerobes, and the CFU recovered on the plates incubated anaerobically are facultative microorganisms that are also being recovered on the plates incubated aerobically. Caution must be exercised, however, when bioburden is being recovered from human or animal tissue or other natural products such as plant-derived materials. Ongoing recovery of strict anaerobes, possibly in high numbers, can occur.
With an injection-molded polymeric device, there’s generally no point in continually testing for anaerobes if strict anaerobes are either not found or found only occasionally in low numbers. Also, the presence of anaerobic conditions doesn’t necessarily mean the presence or potential for growth of strictly anaerobic microorganisms. Products made from absorbable polymers are often stored under dry nitrogen to protect them from oxidative- and/or moisture-related damage. Being a synthetic polymer, contamination with strict anaerobes is highly unlikely and storage in dry nitrogen doesn’t provide an exogenous source of the nutrients and moisture required for microbial growth.
Testing for aerobes and yeasts/molds is always important and the dilution factor can be eliminated by combining the two tests. The entire extraction volume from each of 10 products can be assayed on a general-purpose growth medium, such as soybean casein digest agar. The plates are incubated at 30° to 35°C for 48 hrs., and the incubation continued at 20° to 25°C for an additional 72 hrs., then the plates are counted4. If testing for spores isn’t desired and testing for anaerobes has shown that this testing isn’t indicated or required, a bioburden determination can be performed with this one test, thereby eliminating a dilution factor that can artificially inflate the average bioburden value.
In practical terms, for a test involving 10 products that’s conducted in a manner so there’s no associated dilution factor, the minimal batch average bioburden that should be claimed is 0.3 CFU. This outcome would be from a result of zero CFU recovered in a test that had a recovery efficiency of 1. The conversion of 10 outcomes recorded as “< 1” into an average of 1.0 is not statistically supportable—a finding of zero CFU with an expected value of 10 CFU has a probability of ~4.5 × 10-5. Use of the one-half LOD approach would yield an average bioburden estimate of 0.5 CFU.
Implications of These Approaches
The bioburden data in Table 1 are actual results. In fact, this data set and the conclusion with respect to the average bioburden was reviewed in a recent audit by a Notified Body and challenged as being an overestimation of the true product bioburden and the validity of the associated dose-establishment exercise was seriously questioned.
Drug/device or convergent technology products made in highly controlled manufacturing environments, often with presterilized components, are likely to have very low bioburden. An accurate estimation of the bioburden is important in sterilization process validation studies, particularly with radiation sterilization. With either Method 1 or Method VDmax, higher average bioburden translates to a higher minimum sterilization dose.
Once the challenge has been met with respect to manufacture of a product with low bioburden, an overall methodology must be used to give an accurate determination of the average bioburden. The first instinct would be to perform a traditional bioburden determination as described in Table 1, the testing of 10 products from each of three production batches. A pooled sample, or a most-probable-number (MPN) approach, isn’t common in initially-used methods for bioburden determination. The pooling of product units into one test can obscure item-to-item bioburden variability, and the results of the MPN approach lack information on the bioburden level for any nonsterile product units.
For products with a low average bioburden, the Poisson Distribution-based substitution for the “less than” values gives a bioburden estimate that’s statistically tenable. The one-half LOD substitution is less appropriate in some cases as it leads to an overestimation of the bioburden as the number of product items with no recovered CFU increases. Use of the Poisson Distribution-based substitution approach will yield an average bioburden value that can be carried forward to the “look-up” table in Method 1 or Method VDmax to identify the appropriate radiation dose for the verification dose experiment. Such an average bioburden value, and the consequential verification dose, will have a statistical underpinning and will avoid/withstand technical/regulatory challenge.
References
1. Sterilization of health care products — Radiation — Part 2: Establishing the sterilization dose, ANSI/AAMI/ISO 11137-2:2006, Arlington, VA, Association for the Advancement of Medical Instrumentation, 2006.
2. Sterilization of medical devices — Microbiological methods — Part 1: Determination of a population of microorganisms on products, ANSI/AAMI/ISO 11737-1:2006, Arlington, VA, Association for the Advancement of Medical Instrumentation, 2006.
3. Clarke, JU, “Evaluation of censored data methods to allow statistical comparisons among very small samples with below detection limit observations”, Environ Sci Technol 32:177-183, 1998.
4. Marshall V, Poulson-Cook S, Moldenhauer J, “Comparative mold and yeast recovery analysis (the effect of differing incubation temperature ranges and growth media),” PDA J Pharm Sci Technol, 52(4):165-169, 1998.
Dr. Harry F. Bushar is a statistical consultant for FDA/CDRH/OSB/DBS, where he was previously employed as a mathematical statistician.
Dr. John B. Kowalski is a principal consultant with SteriPro Consulting, a division of Sterigenics International.
Gregg Mosley is an internationally recognized authority in GMP compliance, sterilization, and manufacturing of sterile medical products.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
