Saying Goodbye to a Once-Popular Hysterectomy Device
March 17, 2015
More than a year after cancer concerns were raised, laparoscopic power morcellators have fallen by the wayside.
Chris Newmarker
|
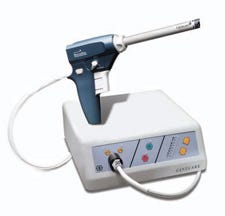
The Gynecare Morcellex Tissue Morcellator, as shown on Ethicon's website. |
Despite worries that limits placed on laparoscopic power morcellators would cause major shifts to open surgery, doctors have found other noninvasive ways to perform hysterectomies, according to The Wall Street Journal.
Citing soon-to-be-published research out of Yale University, the Journal reports that among gynecologists and fellowship faculty members at large U.S. teaching hospitals who performed many of the procedures, 78% have switched away from power morcellators. Most are instead engaging in a "mini-laparotomy" that involves removing the uterus through a tiny incision above the pubic bone.
A mini-laparotomy typically involves an incision 1 ½ to 3 in., while the morcellator procedure incision was generally ½ to ¾ in. The larger incision could increase risk of infections, but it isn't the shift back to open hysterectomies some predicted.
The Wall Street Journal first raised concerns about morcellators in late 2013. By April 2014, FDA had its own warning out. By November, FDA was advising physicians not to use power morcellators to remove the uterus (hysterectomy) or uterine fibroids (myomectomy) from patients who are peri- or post-menopausal, or are candidates for removing tissue intact through the vagina or a minilaparotomy incision.
Over the summer, Johnson & Johnson's Ethicon subsidiary pulled its morcellators from the market, including the Gynecare Morcellex Tissue Morcellator, Morcellex Sigma Tissue Morcellator System and the Gynecare X-tract Tissue Morcellator.
The issues surrounding the morcellators have not only effected the companies that make them, such as Ethicon, but also companies such as Intuitive Surgical, which makes surgical robots for the early stages of hysterectomies, before morcellation takes place.
FDA estimates that 1 in 350 women undergoing either a hysterectomy or myomectomy to treat fibroids has an unsuspected uterine sarcoma, a type of uterine cancer that includes leiomyosarcoma. There is no reliable way to predict or test whether a woman with fibroids may have a uterine sarcoma, the agency said.
"If laparoscopic power morcellation is performed in women with unsuspected uterine sarcoma, there is a risk that the procedure will spread the cancerous tissue within the abdomen and pelvis, significantly worsening the patient's long-term survival," FDA said last year. "While the specific estimate of this risk may not be known with certainty, the FDA believes that the risk is higher than previously understood."
For its part, Minnetonka, MN-based UnitedHealth Group, the nation's largest health insurer, has placed new restrictions on its coverage of hysterectomies.
Refresh your medical device industry knowledge at BIOMEDevice Boston, May 6-7, 2015. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like