The Essure Death Reports in FDA's Database Are Confusing but Tragic
FDA has received 94 reports claiming to link the controversial birth control device to death, but how reliable are those reports?
September 29, 2021
FDA has received nearly 100 reports claiming a link between Bayer's now-discontinued Essure birth control implant and a death, the agency reported on Wednesday.
The problem is, not all of those reports are reliable. In fact, eight have already been determined to be incorrectly coded, dropping the number of total reports from 94 to 86. It's not until you start dissecting the remaining 86 reports, however, that it really gets confusing.
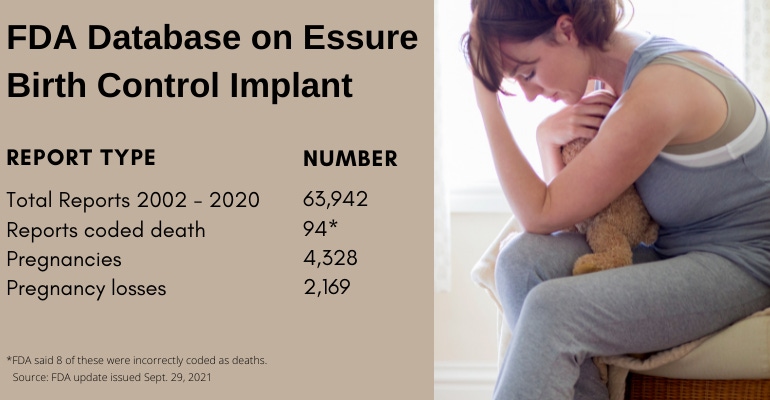
The agency said that 25 reports relate to 23 adult deaths. Another 25 reports relate to 22 incidents of pregnancy loss. Five reports relate to five incidents of a death of an infant after live birth. Two reports relate to two incidences of ectopic pregnancies (a complication of pregnancy in which the fertilized egg attaches outside the uterus). One report specifies a death, but does not indicate whether the death was before or after birth. However, 28 of the Essure reports coded death that FDA received through Dec. 31, 2020 actually just reference information on deaths posted on social media.
"It is difficult for the FDA to determine whether the device caused the death with only the information provided in the report," the agency said in its most recent update, issued September 29.
Essure adverse event reports by the numbers
All told, from the date FDA approved Essure (Nov. 4, 2002) through the date that Bayer stopped selling Essure in the United States (Dec. 31, 2020), FDA received 63,942 medical device reports related to the birth control implant. Most reports received between 2013 and 2015 were voluntary reports submitted from women who received Essure implants. Beginning in 2016, Bayer submitted most of the reports received by FDA. In 2020, Bayer submitted 99% of the reports received by FDA related to the implant.
Broadening the scope, FDA recieved a total of 16,086 adverse event reports related to Essure just in 2020 alone. In 2019, the agency received 15,083 medical device reports related to the device, up from just 6,000 reports submitted in 2018, and the 11,854 reports FDA received in 2017. The nature and severity of the reports in 2020 remain consistent with prior years, the agency said.
It's important to note, however, that 91% of all submitted adverse reports on Essure in 2020 cited litigation. Likewise, in 2019, 95% of submitted reports cited law suits. The agency said that for both 2017 and 2018, 73% of all submitted reports on Essure cited litigation. This is relevant because litigation reports may reference information that was previously submitted to FDA, creating the potential for duplicate reports.
The agency also noted that most of the Essure reports it has received each year since 2017 used terms related to device removal. Specifically, that's 92.7% in 2017; 87.5% in 2018, 91% in 2019, and 93.5% of Essure reports FDA received in 2020 mentioned device removal or related terms.
"The FDA continues to evaluate other sources of postmarket data, including results from mandated postmarket studies, in addition to medical device reports to gather more information about device removals," the agency said.
From 2002 through 2020, the most frequently-reported patient problems associated with the Essure implant were pain/abdominal pain (37,413), heavier menses/hemorrhage/menstrual irregularities (17,332), foreign body/device fragment in patient (9,855), perforation (8,943), headache (8,676), fatigue (7,182), weight fluctuations (6,068), depression/anxiety (5,731), hypersensitivity/rash (5,291), and hair loss (5,084). Most of the reports received listed multiple patient problems in each report.
The most frequent device problems reported about Essure were patient-device incompatibility/biocompatibility, such as a possible nickel allergy, or patient's anatomy related to failure (8,283), migration of the device or device component (5,311), device breakage/material fragmentation/fracture (2,704), dislodged or dislocated device (2,577), device operating differently than expected, for example, implant failure or pregnancy (1,058), malposition of the device (401), device difficult to remove (343), and device difficult to insert (335). Multiple device problems can also be listed in each report, FDA noted.
Thousands of women got the Essure implant, but got pregnant anyway
If you're not confused yet, let's keep digging into the data, turning now to the 4,328 reports of pregnancies in patients with the Essure birth control implant from 2002 through 2020. FDA said some of those reports contained information on multiple pregnancies or more than one pregnancy loss. Of the total pregnancy reports related to Essure, 1,010 were live births, 2,169 were pregnancy losses. And finally, 1,598 of the Essure pregnancy reports did not indicate whether the pregnancy resulted in a live birth or pregnancy loss.
Now, as for those 2,169 pregnancy losses reported: 566 were reported as ectopic pregnancies; 266 were reported as elective abortion; and 1,068 were reported as "other" pregnancy losses. FDA said that due to the limited information provided in these reports, the agency cannot determine whether the pregnancy loss was due to the Essure device in those 2,169 cases.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
