PC Interfacing for the Medical Market: Is a New Standard Emerging?
February 1, 1999
Medical Device & Diagnostic Industry Magazine
MDDI Article Index
An MD&DI February 1999 Column
 MEDICAL ELECTRONICS
MEDICAL ELECTRONICS

The development of high-speed serial buses has prompted the need for a specification to facilitate system expansion for the custom peripherals that are critical to fast-evolving medical device technology.
It is an unmistakable trend that personal computers (PCs) have become the brains behind many medical equipment systems, just as they are dominating a range of other embedded applications. Over the past decade, PCs have replaced proprietary designs in virtually every area of medical equipment, including CT-scan machines, ultrasound equipment, ECG and x-ray units, and numerous others. PCs have found their way into the medical market for several reasons. As the current standard for business applications, PCs offer the best price and performance value and a wider variety of development tools, operating systems, and peripherals than any other platform.
BUS INTERFACE TECHNOLOGY
One constant in the evolution of the computer industry has been the call for increasingly higher bandwidths in bus interface technology. In the early days, when the XT was the PC standard, the 8-bit ISA bus was the standard for peripheral interface. With the advent of the AT, the 8-bit ISA bus was found to be short on resources and not fast enough, so the 16-bit ISA bus was born. This bus ran at the same clock frequency, but its data path was twice as wide. As the 16-bit ISA bus in turn became too slow for the processing power in the computer, other standards began to appear that had varying degrees of success.
The EISA and VESA buses were the first to be popular in the commodity computer market for expansion. Although both of these buses were enhancements of the existing 16-bit ISA bus and took bus interfacing to a higher level, they were short-lived and never truly gained popularity in embedded applications. Shortly after the introduction of EISA and VESA, PCI came along, offering more bandwidth and features. The currently used PCI bus has a 32-bit data bus and a clock frequency more than four times faster than that of the ISA bus. However, because many medical I/O applications don't require the performance of PCI, manufacturers of medical equipment continued to produce ISA-designed cards, which are relatively easy to customize and cost less than PCI components.
For several years, computers have been shipping with both PCI and ISA bus slots. These slots have given developers of industrial and medical applications the opportunity to leverage the high-volume, commodity PC platform as the basis of their control system. By using an off-the-shelf, standard PC, medical-control-system designers are dramatically reducing overall design time and costs. Buying most of the hardware and the operating system off the shelf can help the system designer achieve a shorter design cycle, which aids in getting the product to market quickly.
Using a commodity computer platform is not without problems, however. As the commodity market tries to make the computer experience easier for the user, some of the legacy bus interfaces fail to fit the envisioned model. The ISA bus is not particularly user-friendly, and raises a number of support issues. Because ISA peripherals are not fully plug-and-play compatible, the user must assign the resources of the card at the time of installation. During the configuration process, the installer must know which resources the peripheral uses as well as the resources used by all other components in the system.
It would thus seem logical that PCI would become the standard for PC expansion for the near future. Given the high bandwidth and plug-and-play nature of PCI, many of the problems characteristic of ISA do not exist in the PCI standards. As PC peripherals continue to require more and more bandwidth, PCI has kept up by beginning to migrate from the current 32-bit, 33-MHz version to a higher-performance, 64-bit, 66-MHz version.
HIGH-SPEED SERIAL BUSES
There are significant disadvantages associated with designing to the PCI bus, however. First, PCI is much more complex and less forgiving than ISA. This makes new peripherals more complex and expensive to design as well as to manufacture. In addition, the 33-MHz version of PCI typically limits the available expansion in a system to four slots or less, with the 66-MHz version offering only two slots. As a result of these inherent problems with the PCI bus, a new generation of high-speed serial buses—USB and IEEE 1394—has been created. These new serial buses are intended to make new peripheral designs easy to develop, inexpensive to manufacture, and simple to install and service. The peripherals can also be deterministic, unlike what is possible with other serial communication standards such as Ethernet. As such, they promise to eliminate a number of potential problems in both medical and business applications.
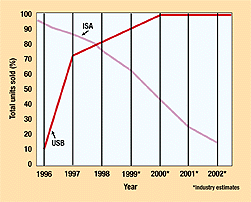 Figure 1. The percentages of ISA- and USB-enabled PCs sold in the past two years show a trend in favor of the newer bus. Data retrieved from USB Technology and Market Report (San Diego: Annabooks, October 1998).
Figure 1. The percentages of ISA- and USB-enabled PCs sold in the past two years show a trend in favor of the newer bus. Data retrieved from USB Technology and Market Report (San Diego: Annabooks, October 1998).
USB (Universal Serial Bus) is an interface that has been widely available on the PC platform since 1996. The USB Specification V1.1 defines the physical layer and protocol for a medium-bandwidth serial bus operating at 1.5 or 12 Mb/sec. This hot-swappable, plug-and-play, half-duplex serial bus began to achieve widespread acceptance at the end of 1998 (as was apparent at the fall 1998 Comdex show in Las Vegas)(Figure 1). USB permits nearly unlimited expansion by allowing a single externally accessible port to support up to 127 peripherals.
With USB, expanding the PC became as straightforward as plugging a cable into a running computer. Following insertion of a four-wire cable into the USB port on the computer, the operating system enables the device and loads the drivers, so that the peripheral becomes available for use almost instantly. Commonly available commodity USB devices are mice, floppy drives, speakers, and keyboards, to name a few. In the industrial and medical markets, common uses of USB include instrumentation applications such as digital and analog I/O systems.
Complementary to USB is a higher-speed serial bus, IEEE 1394, commonly referred to as 1394. IEEE 1394 is a hot-swappable, plug-and-play, full-duplex serial bus. The current version of 1394—known as 1394-1995—was ratified by IEEE in December 1995 and allows for transfer speeds up to 400 Mb/sec (Figure 2). Version 1394b is currently in draft form and will provide for speeds up to 3.2 Gb/sec. Although 1394b devices are not yet available, vendors are already shipping devices capable of using the full 400-Mb/sec bandwidth. With these faster speeds permitting throughput higher than that of standard PCI buses, 1394 has found a place in applications starved for bandwidth. A new approach to device interfacing, 1394 can feature up to 63 devices with a peer-to-peer relationship on a single bus. Like SCSI, 1394 devices can communicate directly with one another, eliminating the need for the computer to arbitrate communication; in fact, even with the computer off, most 1394 implementations allow the peripherals to continue to operate. Among the current uses benefiting from 1394 are video and imaging applications.

Figure 2. Comparative maximum data-transfer rates of ISA, PCI, and IEEE 1394 buses.
Although USB and 1394 are rich with technological advances, a few implementation issues present difficulties for certain industrial and medical applications. One is the issue of surprise removal of devices. Because USB and 1394 are hot-swappable and plug-and-play by nature, a user may decide at any time to remove the cable that connects the device to the computer. This could happen at an inopportune moment—for example, during a motion operation or during data acquisition. Such surprise removal could cause a catastrophic failure in a control or instrumentation system, rendering the machine useless.
Another implementation problem with USB and 1394 in industrial and medical applications concerns the power required for the device. Though both the USB and the 1394 cable can provide power to devices, the use of this power is limited to low-current devices using a single voltage rail. Most industrial and medical applications require a relatively large amount of current at several voltage levels, and often this power must be clean. Most developers would therefore choose to design in power supplies or to provide power from an external power supply, increasing system cost and complexity.
A final implementation difficulty has to do with mechanical issues. Neither USB nor 1394 has an associated specified mechanical enclosure for devices. The problem this creates is that every device in the control system could conceivably be a different size and have a separate set of requirements for mounting to the machine. This can render service difficult, because the machine may need significant disassembly to remove damaged components from the chassis. Upgrading also becomes more complicated, since changes in mechanical dimensions may necessitate housing and panel redesign.
Feature | ISA | PCI | USB | IEEE 1394 | Device Bay |
|---|---|---|---|---|---|
Parallel interface | Up to 16 bits | Up to 64 bits | — | — | — |
Serial interface | — | — | Yes | Yes | Yes |
PC99 compliant | No | Yes | Yes | Yes | Yes |
Can be installed inside computer | Yes | Yes | Yes | Yes | Yes |
Can be installed outside computer | No | No | Yes | Yes | Yes |
Isochronous transfers | No | No | Yes | Yes | Yes |
Fair-access policy | No | No | Yes | Yes | Yes |
Plug-and-play compatible | No | Yes | Yes | Yes | Yes |
Power management | No | Yes | Yes | Yes | Yes |
CRC/Data checking | No | No | Yes | Yes | Yes |
Defined mechanical form factor | Yes | Yes | No | No | Yes |
Hot-swappable | No | No | Yes | Yes | Yes |
Surprise removal allowed | No | No | Yes | Yes | No |
Voltages supported | ±5V, ±12V | 3.3V, 5V, ±12V | 5V | 8V to 40V | 3.3V, 5V, 12V |
Expansion | 1 to 5 slots typical | 4 slots typical (33-MHz) | Up to 127 | Up to 63 | Up to 127 USB devices |
Table I. PC bus feature comparison.
THE DEVICE BAY STANDARD
In order to solve the aforementioned implementation problems and to open USB and 1394 to a wider gamut of devices, a new industry standard has recently emerged and is building some momentum in the embedded-computer market. Authored by Compaq, Intel, and Microsoft, the Device Bay specification enhances the USB and 1394 specifications (Table I). Device Bay is the electrical and mechanical form factor for the USB and 1394 high-speed serial buses, and is designed to allow hot-swappable, plug-and-play expansion for computer peripherals. The Device Bay standard allows for typical computer devices like hard drives, CD-ROMs, and DVDs to coexist on the same bus with custom-designed, embedded devices that perform, for example, patient monitoring or medical system control.
The Device Bay standard allows for three physical options. The largest of the three resembles a 5¼-in. hard drive in its dimensions. Referred to in the Device Bay specification as a DB-32, this form factor—the only one currently in production—measures 1.26 in. high X 5.75 in. wide X 7.01 in. deep. Device Bay incorporates a single connector that supports both USB and 1394 and provides additional power to accommodate more power-hungry devices.
Commodity PCs have become very attractive for many applications that require the latest computer technology. Previously, designing in the latest technology required a large amount of research to ensure that the proprietary portions of the system were compatible with the other resources of the computer. By its nature, Device Bay is inherently portable; any computer that has a USB port, a 1394 port, and a Device Bay–compatible operating system is capable of using Device Bay technology. If the devices work on one system with a particular configuration, they will work on all platforms.
Unlike ISA and PCI, USB and 1394 devices require no system resources: they do not rely on the PC's I/O or memory map, interrupts, or DMA channels. In the past, these legacy issues have prevented system designers from developing complex systems. Systems were limited in functionality because the designer often ran out of resources in the base platform before implementing all of the necessary features.
In many designs, the specifications change during the life of the product in order to keep it competitive in the market. Currently, designers do their best to guess the amount of expansion that will be necessary over the product's life, and try to design it in to prevent complete system redesigns. Providing such expandability in advance is not only costly, it often goes unused. If, on the other hand, the designer underestimates the need for expansion, expensive system redesign is the only option.
An ongoing concern for medical systems designers has been the problem of maintaining certifications when using commodity PC components. Many times manufacturers of computer components—such as hard drives or video cards—will discontinue components faster than the system designer can get certifications done. With the Device Bay technology, two separate subsystems that are independently certified make up the computer system.
The first is the computer itself. The computer will contain the processor, RAM, video, and possibly a hard drive. This type of stripped-down computer—commonly known as a Net PC or "legacy-free" PC—will typically carry certifications from the manufacturer such as CE, UL, and CSA designations. Because the computer carries its own set of certifications, it becomes a "component" that can be replaced by another computer marked with the same set of certifications.
The second subsystem would be Device Bay–based and contain all of the application's proprietary components and other interfaces, such as a floppy drive or CD-ROM. Since the medical system manufacturer controls the manufacturing of the proprietary components, this portion of the system will remain constant. Changes made to this second subsystem can be anticipated and planned in advance.
VIRTUAL INSTRUMENTATION: One vision of the future of medical devices entails eliminating the need for multiple pieces of equipment through "virtual instrumentation." Each hospital room could be equipped, for example, with a low-cost Net PC, a display (LCD touch panel or CRT monitor), and a Device Bay condo. Whenever a particular instrument is needed, a Device Bay module containing the specific instrumentation electronics would be installed into the condo. The PC would recognize that a device had been inserted, automatically load the appropriate drivers, and in essence transform the computer system into the instrument. For instance, a patient in a hospital room might require blood-pressure monitoring, ECG monitoring, and blood-oxygen analysis. Normally this would require three separate pieces of equipment. With Device Bay, however, a medical technician could simply insert a separate module for each function into the condo. The application software would automatically load the required drivers and run and display the desired application. All patient testing and monitoring would be run from a single PC, with all patient data displayed on the same screen. Medical personnel could use a mouse, a touch screen, or a keyboard to set up the instruments to certain parameters such as alarm limits, data-logging information, etc. Because the PC would be networked to a central computer, all patient data could be saved and monitored at a main nursing station, including alarm information. The patient's medical data and medical history would also be available to medical personnel on-line in the patient's room. Although another patient in another room may require no tests or monitoring, the system could still be used for functions such as retrieving medical charts or x-rays. The versatility of such an open, modular system is virtually unlimited. As more computer power is required, only the computer itself would need to be updated, not the complete system. As more devices become available or are improved, only the device modules would change. Because this is an open standard, modules from multiple vendors could coexist in the same system. This would allow the hospital to buy the best device for each type of instrument without having to be locked into one particular vendor. |
APPLICATIONS FOR MEDICAL EQUIPMENT
What would a medical device, such as an ECG or an ultrasound machine, that incorporated Device Bay technology look like? The following example describes a stand-alone piece of medical diagnostic equipment. Inside the chassis would be a low-cost PC such as a Net PC. A Device Bay condo with four bays would be accessible from the front, connected to the embedded PC through the USB and IEEE 1394 connections. For the display, an LCD panel or standard VGA monitor can be used. Pointing-device options include a touch screen, mouse, keyboard, or a combination of these; if not installed initially, the mouse and keyboard can be added at any time through the USB walk-up port. This is the basic construction of the machine, which is generic in nature and can be tailored to meet a variety of needs.
 With Device Bay technology, removing a device is as straightforward as removing a tape from a VCR. Security features operated through a combination of software and hardware prevent surprise removal of devices and potential data loss.
With Device Bay technology, removing a device is as straightforward as removing a tape from a VCR. Security features operated through a combination of software and hardware prevent surprise removal of devices and potential data loss.
For an ECG machine, current ISA or PCI designs would be ported to Device Bay modules. The modules would have an on-board, low-cost microprocessor to communicate with the Device Bay controller and handle the communications for the USB or IEEE 1394 interface. This microprocessor would also be used to add local intelligence to the device: in this example, it could initialize the high-speed A/Ds, perform a power-up self-test, and handle background functions such as polling the A/D channels and signal conditioning the inputs. The microprocessor can also compress, time-stamp, and organize the data in a logical way—sparing the PC from having to perform these functions.
Because the computer platform was previously configured to use the ECG module, the module can be inserted into any available bay within the base instrument. Since Device Bay is plug-and-play, the unit can already be powered up when the module is inserted. The operating system and application will immediately recognize the device, load appropriate drivers, and run the ECG application. Recorded information can be logged through a network interface, a hard drive, or a read/writeable CD.
A USB or 1394 camera can be added to visually record the patient and synchronize these images with the ECG data. This would, for example, eliminate the need to have a medical technician mark the chart whenever a patient coughs or makes other movements. Installing this option would merely require plugging the camera into an available USB or IEEE 1394 walk-up port. Data from the camera could be incorporated with the ECG test record.
This roughly sketched example illustrates how a low-cost computer with Device Bay can very much simplify a piece of medical test equipment. The modularity of the architecture design offers flexibility and expandability while reducing the system design cycle and simplifying its manufacture.
CONCLUSION
Medical equipment continually requires more sophisticated computer performance. PCs can fulfill this thirst for performance and are the most cost-effective way to implement computer power. However, as this article illustrates, PCs have been something of a moving target for medical OEMs, who try to maintain consistency and longevity of components within their products. Older PC technology brings in a variety of legacy issues that make these older systems difficult to implement and expand in a dedicated environment. Device Bay represents one practical and long-awaited solution to these shortcomings.
USB and IEEE 1394 are already replacing ISA devices and soon could be replacing PCI in many applications, including medical devices. By providing the standard needed to integrate these relatively new technologies into practical medical applications, Device Bay offers system designers numerous new opportunities to make their products better by making them easier to manufacture, upgrade, and service.
Kent Tabor is president and Clint Hanson is director of hardware engineering at Granite Microsystems (Mequon, WI), an engineering and manufacturing company that specializes in medical and industrial embedded OEM computer solutions.
For More Information on PC Interface Standards Device Bay Web site—http://www.device-bay.org USB Web site—http://www.usb.org 1394TA Web site—http://www.1394ta.org PCI SIG Web site—http://www.pcisig.com PC 99 Specification—http://www.microsoft.com/hwdev/xpapers/pc99/default.htm USB Specification V1.1—http://www.usb.org/developers/index.html IEEE 1394-1995—http://www.standards.ieee.org/catalog/bus.html#1394-1995 |
Photo by Roni Ramos
Copyright ©1999 Medical Device & Diagnostic Industry
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
