FDA Approves Edwards ClearSight Noninvasive Blood Monitor
June 30, 2014
|
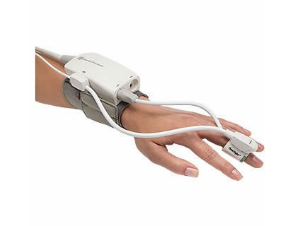
Edwards' ClearSight noninvasive blood monitor (Courtesy Edwards Lifesciences) |
Edwards Lifesciences (Irvine, CA) has announced that it has received FDA 510(k) approval for its ClearSight noninvasive monitor that, in conjunction with the company's EV1000 clinical platform, provides blood volume and blood flow information for patients at moderate or high risk of post-surgical complications, for whom invasive monitoring would not be used.
Edwards says the ClearSight system is the most advanced noninvasive monitor of its kind, The system incorporates a finger cuff on the outside of the finger and software elements that have been used for the noninvasive monitoring of the blood pressure of astronauts in space.
The inflatable finger cuff provides access to key hemodynamic parameters, including Stroke Volume (SV), Stroke Volume Variation (SVV), Cardiac Output (CO), Systemic Vascular Resistance (SVR), and Continuous Blood Pressure (cBP).
The ClearSight system automatically provides up-to-the-minute information without inserting anything into the body. The system thus extends the benefits of hemodynamic optimization, or proper fluid administration and balance within a patient's organs and tissues, to a broader patient population that could benefit from close monitoring, but may not receive it without a noninvasive option.
"Proper intraoperative management of moderate and high-risk surgery patients is critical to help reduce the risk of post-surgical complications," said Julie K. Thacker, MD, surgical oncologist at Duke University Hospital. "Studies have indicated that patient outcomes are improved through monitoring and management of vital hemodynamic information through hemodynamic optimization protocols."
Refresh your medical device industry knowledge at MEDevice San Diego, September 10-11, 2014. |
The EV1000 clinical platform allows clinicians to set specific color target ranges to clearly indicate patient status; and to choose parameters, alarms and targets to align with hemodynamic optimization protocols to meet precise patient monitoring needs. Edwards cites clinical data that demonstrate noninvasive ccNexfin BP technology obtains mean arterial pressure values comparable to invasive monitoring with the advantage of continuous measurement.
Several studies have shown the ability of this noninvasive technology to measure not only absolute cardiac output values, but also reliably track real-time changes in continuous cardiac output (CO). The ClearSight system provides valuable hemodynamic insight into an expanded patient population, allowing clinicians to make more informed decisions about volume administration in moderate to high-risk surgery.
The ClearSight system has also been approved in CE Mark countries.
Stephen Levy is a contributor to Qmed and MPMN.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
