FDA Approves Artificial Cartilage Implant
July 8, 2016
Made out of polyvinyl alcohol (PVA), the Cartiva Synthetic Cartilage Implant (SCI) is meant to replace the damaged cartilage surface of the big toe.
Qmed Staff
|
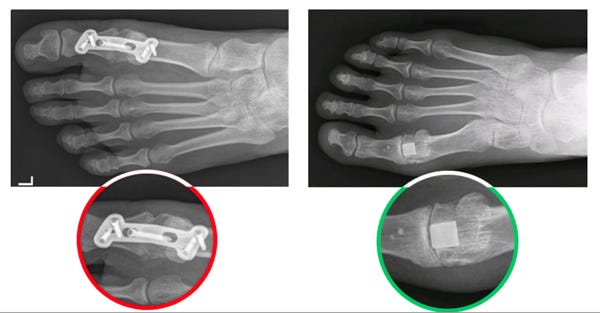
The results of a big toe joint fusion procedure (left) versus implanting of the Cartiva SCI (right). (Image courtesy of Cartiva) |
Cartiva (Alpharetta, GA) recently announced what it is touting as the first U.S. FDA approval of a synthetic cartilage device, the company's Cartiva Synthetic Cartilage Implant (SCI) for arthritis of the big toe joint.
The implantable device, which is made out of biocompatible polyvinyl alcohol (PVA), is meant to provide relief of arthritis at the base of the big toe, replacing the movement-limiting joint fusion procedures that have been used to treat the painful condition.
The Cartiva implant is designed to have physical properties similar to those of articular cartilage, including compressible, low-friction and durable bearing surface.
FDA's decision came after a 236-patient, multi-center, randomized clinical study that compared Cartiva SCI to fusion. The Cartiva SCI ended up having a 80% success rate in pain, function, and safety, versus 79% for the fusion group. Cartiva patients also saw a nearly three-time improvement in median sporting activities function, and 65% improvement in daily living activities. Cartiva patients saw their range of motion improve 26% from baseline.
"The landmark Motion study clearly shows Cartiva SCI to be a safe and effective alternative to fusion for patients wishing to maintain motion in their great toe," said Judith Baumhauer, MD, a University of Rochester Medical Center professor and principal investigator of the Motion study.
Founded in 2011, Cartiva is venture capital backed. Its LinkedIn profile lists 11 to 50 employees. The company's mission has been to develop innovative treatments for what it says are an estimated 27 million Americans who suffer from osteoarthritis.
Chris Newmarker is senior editor of Qmed. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like