EU MDR Is the New FDA PMA
A new CE mark for an oxygen machine serves as a reminder that passing muster under the EU's Medical Device Regulation is no small feat.
August 23, 2023
Remember when it was a widely accepted strategy to bring new medical devices to the European market first, and then pursue the taller regulatory hurdle known as FDA approval? The new Medical Device Regulation (MDR) in the European Union is expected to turn that strategy on its head.
That said, some U.S. based medical device companies are beginning to report CE marks under the new MDR, which is an optimistic sign for what the European medical device market will look like after the new regulation is fully implemented.
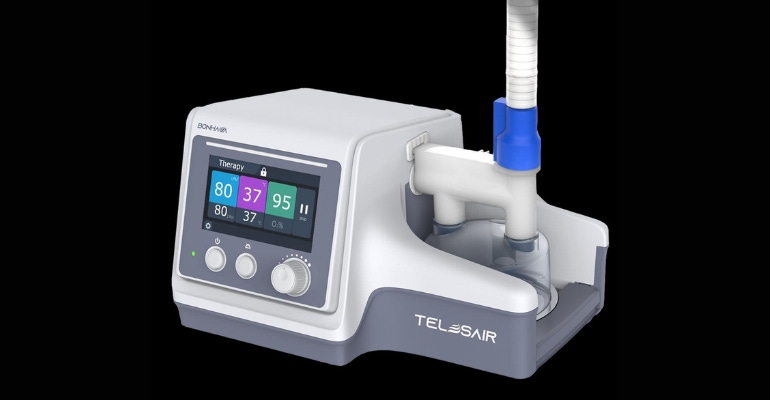
One recent example is Telesair.
Based in Irvine, CA, Telesair recently received CE marking under the MDR for its Bonhawa high flow oxygen therapy system (HFOT) for treating patients with respiratory insufficiency. Telesair said its Bonhawa HFOT is one of the first respiratory therapeutic devices from a U.S. based company to receive CE mark under the new Medical Device Regulation.
The company touts that the Bonhawa HFOT is light and compact and features an extended flow range up to 80 L/pm, a simple disinfection process and an easy-to-use touchscreen, allowing greater therapeutic range, efficient disinfection, the potential to reduce the workload of caregivers, and the ability to visualize patient settings and data from outside the room.
“Having our Bonhawa system approved for Europe is a major step in the evolution of Telesair as a company and opens the many markets which accept CE mark,” said CEO Bryan Liu.
Liu said the company accomplished the goal in less than 10 months.
“We are already working on positioning our next generation home-based platform which we also intend to get approved swiftly,” Liu said.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
