Dexcom's Bid for Non-Adjunctive Label Blessed by Panel
An FDA advisory committee voted in support of a non-adjunctive indication for Dexcom's G5 Mobile Continuous Glucose Monitoring system.
July 22, 2016
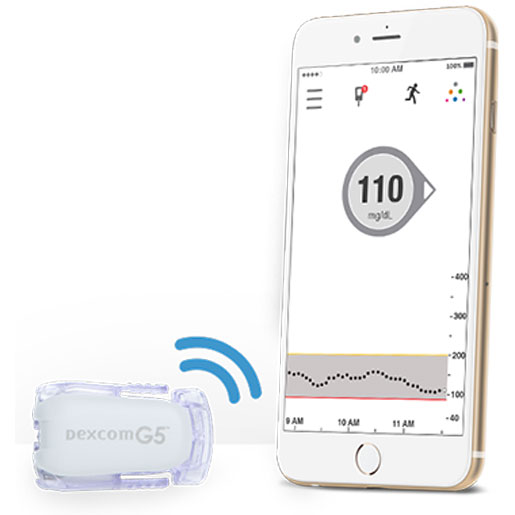
Dexcom got a big vote of confidence from an FDA advisory committee Thursday. The major maker of continuous glucose monitoring (CGM) systems for patients with diabetes is pursuing a non-adjunctive indication from FDA for its G5 Mobile CGM system. That indication would allow the G5 Mobile CGM system to be labeled and marketed for use in making insulin dosing and treatment decisions.
The system currently is designated in the United States for adjunctive use, meaning patients are supposed to use fingerstick glucose testing for confirmation before making treatment decisions. Dexcom has CE Mark approval for non-adjunctive labeling.
Thursday, an FDA advisory committee--the Clinical Chemistry and Clinical Toxicology Devices Panel--voted 8-2 that the benefits of the G5 Mobile CGM system outweigh the risks for the proposed non-adjunctive indication. The panel also gave favorable votes to FDA's questions of whether there is reasonable assurance that the proposed indication is safe (8-2 vote) and effective (9-1 vote).
"We have always believed that the information provided by CGM is much better for making an insulin dosing decision than a single fingerstick, particularly as we look at the accuracy and performance of our system with our 505 software algorithm," said Kevin Sayer, Dexcom president and CEO, in a conference call Friday morning.
Start planning your fall schedule now. Register for the MD&M Minneapolis Conference, September 21-22. |
FDA is expected to consider the panel's recommendation when making a decision on the proposed indication. The agency often--but not always--follows panel recommendations.
Dexcom expects to continue communicating with FDA over the coming months on the proposed indication and plans for a postmarket study.
If FDA grants Dexcom approval for the non-adjunctive indication, the company would be able to market and offer training on how to use the CGM system in making treatment decisions.
BTIG analyst Sean Lavin, MD wrote in a July 21 research note following the panel vote, "The elephant in the room was that people are dosing on CGM readings already. By not granting the label for nonadjunctive use, the FDA would be hamstringing the ability of DXCM and providers to educate these patients in making better decisions."
Another reason a non-adjunctive label would be a win is that it is considered a necessary step toward gaining Medicare reimbursement coverage for CGMs. The technology is not covered now because of its adjunctive designation. If FDA approves the non-adjunctive indication, Dexcom will be able to apply for CMS reimbursement coverage, including a designation as durable medical equipment (DME), Sayer said. Cautioning that the process is "inherently unpredictable," he added that the company does not expect a CMS national coverage determination before 2018.
"Today, we have patients who have successfully managed their diabetes with CGM for many, many years. They turn 65 and then they lose coverage. They suddenly face a difficult decision--pay out of pocket or revert to fingersticks--and many times this decision is dictated solely by a patient's financial situation and they are unable to continue with CGM," Sayer said.
Kelly Close, founder of advocacy group The DiaTribe Foundation, told MD+DI, "I am elated that the FDA voted that the benefits outweigh the risks, particularly because the advisory committee took into account how patients perceive both risk and benefit 'in the real world.' While FDA advisory committees tend to focus on downside risk, it was great to see recognition that patients face real-world risks right now, that this label update can mitigate some of those risks, and there will be public health upside to approving this label."
There was an outpouring of public support for the effort at the panel meeting, Close added. In addition to numerous testimonials in front of the committee, a diatribe.org public letter to FDA received almost 10,000 signatures of support and a letter from the Diabetes Patient Advocacy Coalition received thousands of signatures, she pointed out. "I strongly believe that these actions informed the panel's favorable opinion," Close said.
"This recommendation is a big milestone for people with diabetes," Sayer said in a press release. "The diabetes community turned out in force to support this decision. We commend the FDA for bringing this important subject into a public forum, and thank the panel members, as well as the public speakers for their willingness to participate. We look forward to continued positive discussions with the FDA as we seek the agency's approval of our application."
[Image courtesy of DEXCOM]
About the Author(s)
You May Also Like


