Complying with ISO 13485:2016's New Expectations for Supplier Management
Changes to ISO 13485 are impacting supplier management. Are you prepared?
December 18, 2017

One of the main changes to ISO 13485:2016 “Medical devices -- Quality management systems – Requirements for regulatory purposes” pertaining to suppliers is the “increased emphasis on use of a risk-based approach to supplier management,” Ron Schmitz, president of Grand Avenue Software, told MD+DI. “Medical device companies are increasingly required to not only analyze supplier-related risks during supplier qualification, but also to monitor their suppliers’ performance and feed the monitoring results back to a requalification process.”
Grand Avenue Software will be exhibiting at Booth #1612 at MD&M West 2018 in Anaheim February 6-8, 2018.
Schmitz highlighted some of the changes that were introduced within Section 7.4 Purchasing of the 2016 standard. “Per this section, suppliers are selected according to criteria that are based on performance and ability to deliver, as well as on the overall effect of the purchased product or service on the quality of the medical device. A new area of emphasis is that these criteria must be in proportion to the risks associated with the medical device.”
Companies across the medical product supply chain now need to work towards compliance, Schmitz said. “For all involved, that will include a review of current practices versus the requirements, followed by new practices and possibly new tools to close any gaps.”
To help companies comply, Grand Avenue Software has added a new Supplier Management module to its software suite. The web-based platform can be hosted in a secure Tier III data center or deployed on-premise on a company’s own servers. “The user experience is completely available from standard web browsers on both desktop and mobile devices, allowing users to access the system when visiting suppliers,” he says. “And companies may optionally allow their suppliers to participate in the same system so, for example, suppliers can easily train on new documents, implement and verify corrective actions, or get notified of revisions to existing documents.”
The Grand Avenue Supplier Management module includes automated workflows for supplier evaluation and approval. The module also allows companies to dynamically generate their Approved Supplier Lists (ASL) based on current supplier status, eliminating the maintenance of updating a static ASL as suppliers are qualified, updated, and removed. Detailed supplier histories are maintained by the system, helping companies easily find and see related CAPAs, nonconformities, and audits for each supplier.
Schmitz said that historically many companies kept track of supplier activity by manually entering information into spreadsheets. “It was difficult in that environment to keep track of what was needed from each supplier and when. The new standard heightens the need for effective tools to manage supplier information.”
Schmitz said that ISO 13485 changed to adapt to evolving trends in the marketplace. “Unlike when the 2003 version of ISO 13485 was published, almost any aspect of taking a medical device to market can now be outsourced,” he said. “Even though it is common today for medical device manufacturers to leverage design, production, and service suppliers from around the globe, device manufacturers are ultimately responsible for product quality.”
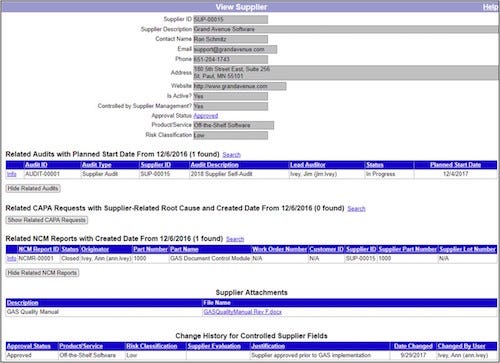
Above: Example of Supplier Performance/History
Please visit Grand Avenue Software at Booth #1612 at MD&M West February 6-8 for more details.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
