Boston Scientific LithoVue Elite Gets FDA Nod
The system is designed to allow urologists the ability to make more informed pressure-related clinical decisions during ureteroscopy.
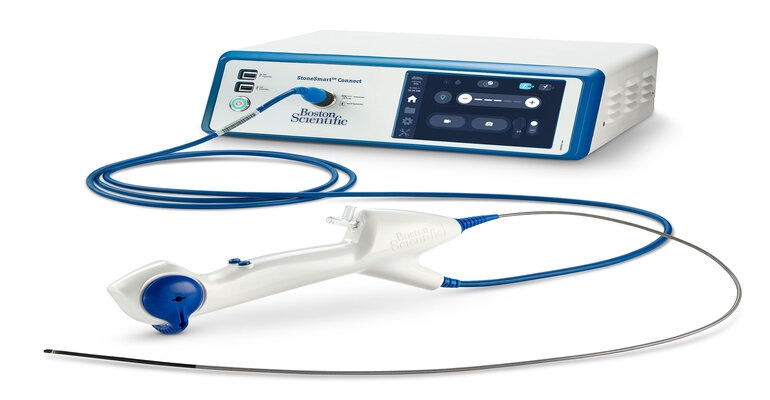
Boston Scientific’s LithoVue Elite Single-Use Digital Flexible Ureteroscope System recently obtained FDA 510(k) clearance, becoming the first ureteroscope system with the ability to monitor intrarenal pressure in real-time during ureteroscopy procedures, according to the company. Ureteroscopy is a common procedure performed to diagnose and treat problems in the urinary tract, like kidney stones, which research shows about one in 10 people will develop at some point in their lives.
During ureteroscopies, elevated intrarenal pressure (IRP) can occur in response to fluid irrigation used to maintain a clear visual field. Of note, high IRP can contribute to post-operative complications including systemic inflammatory response syndrome (SIRS), sepsis, and renal damage. LithoVue Elite’s ureteroscope tip is equipped with a built-in pressure sensor designed to allow urologists to make more informed pressure-related clinical decisions.
"Multiple studies have shown the importance of understanding intrarenal pressure during ureteroscopy procedures to mitigate potential complications, but until now, urologists have lacked an easy way to measure pressure in real-time," said Ben Chew, MD, MSc, associate professor, University of British Columbia, in the press release announcing the clearance. "This device represents an important and needed advancement in single-use ureteroscopes, which could help improve patient care and potentially lead to a clearer understanding of the impact of elevated intrarenal pressure on patient outcomes."
The system is comprised of Boston Scientific’s single-use digital flexible ureteroscope and StoneSmart Connect Console and builds on many of the original LithoVue system’s features with next-generation innovation. Additional to its IRP monitoring, Elite includes upgraded image quality using a high-resolution digital chip and advanced imaging software designed to provide faster, sharper images and create greater depth of field compared to the original system. There are updated control features on the Elite system, including two programmable buttons on the lightweight, single use ureteroscope handle that enables direct control of both videos and images without the need to coordinate with staff. The StoneSmart Connect Console, the system’s compact processing unit, works with existing operating room visualization towers and endoscopic monitors to reduce clutter and capital footprint, according to Boston Scientific.
As the first device built on the company’s next-generation StoneSmart Technology platform, it is intended to help support potential interoperability with future portfolio devices. The limited market release for LithoVue Elite in the United States is reported to begin in the coming weeks.
“We designed the next-generation LithoVue Elite System to bring a greater level of precision and functionality in the way urologists diagnose and treat kidney stones, an increasingly common condition among adults,” said Meghan Scanlon, senior vice president and president, urology, Boston Scientific, in the press release. "We are committed to developing clinical solutions, like the LithoVue Elite System, that are intended to make procedures more efficient, empower surgical decisions, optimize patient care, and ultimately improve the treatment of kidney stone disease globally."
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
