Abbott Absorb Stent Approved by FDA
July 5, 2016
Fully absorbing cardiac stent delivers scar-prevention drug, dissolves after three years.
Nancy Crotti
|
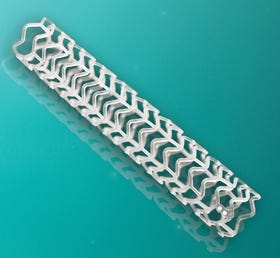
Abbott spent roughly 15 years developing Absorb. (Image courtesy of Abbott Labs) |
FDA has approved Abbott Laboratories' fully resorbable cardiac stent, less than a year after Boston Scientific won the agency's approval for a similar device.
Abbott's Absorb GT1 Bioresorbable Vascular Scaffold System releases the drug everolimus to limit the growth of scar tissue, and is gradually absorbed by the body in approximately three years, according to an FDA statement.
Abbott is claiming that the Absorb is the first fully resorbable cardiac stent, although Boston Sci says its Synergy stent dissolves even faster, in about three months. Abbott has spent some 15 years developing Absorb, and physicians had been anxiously following clinical trials related to the device, which promised to address some of the complications of heart patients treated with stents, including thrombosis and restenosis.
An FDA advisory committee evaluated data from a randomized trial of 2008 patients, which compared the rate of major adverse cardiac events between the Absorb and a drug-eluting metallic stent. The committee voted in March that the benefits of Abbott's bioabsorbable cardiac stent outweigh the risks, despite some safety reservations. A 2015 clinical trial found that the Absorb technology was merely "noninferior" to Abbott's existing Xience stent.
The Absorb performed slightly worse in a clinical trial than the Xience in terms of preventing deaths, heart attacks, and the need for repeat stent procedures. The rate of such events was 7.8% for Absorb patients and 6.1% for the control group. The rate of blood clots forming within the devices was 1.54% for the Absorb and 0.74% rate for the Xience.
While stents are traditionally made of metal, the Absorb stent is made of poly(L-lactide), a naturally dissolving material, similar to dissolving sutures. By contrast, metal stents are permanent implants that restrict vessel motion for the life of the patient. Scar tissue can form within a stent causing the artery to narrow again. Drug-eluting stents temporarily release a drug, typically for a few months after stent placement, to combat the formation of scar tissue.
After absorption, four small platinum markers remain embedded in the arterial walls, which help cardiologists identify where the stent was originally placed, according to FDA.
"No metal means the treated artery can pulse and flex naturally as demands on the heart change with everyday activities," said Gregg Stone, MD, director of cardiovascular research and education, Center for Interventional Vascular Therapy at Columbia University Medical Center and New York-Presbyterian Hospital, in a company statement. "No metal may also reduce the potential of future blockages that occur with permanent metallic stents, and allows easier access to other treatment options should they prove necessary in the patient's future."
Stone chaired the Absorb clinical trial program. Abbott Vascular makes the Absorb stent at its facility in Santa Clara, CA. The company plans to offer the device to hospitals in the United States, starting with interventional cardiology centers that participated in Absorb clinical trials.
FDA beat an estimate by an RBC Capital analyst that the agency would issue a PMA for the device in the fourth quarter of 2016. Abbott's stock had dipped by .58% by 1 p.m. Eastern time.
Nancy Crotti is a contributor to Qmed.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like