Medical Device Marking and Labeling
The IEC 60601-1 standard provides comprehensive requirements for medical device marking and labeling.
Medical device labeling is considered as important as classifying a product or creating an insulation diagram. In IEC 60601-1, labeling is deemed "a critical component of a medical device."1 The standard provides comprehensive requirements for medical device marking and labeling. All information for safe installation, use, storage, servicing, and maintenance of the device must be provided to the user. Some labeling simply reiterates common sense; however, some information is safety-critical--imagine powering up a 120-V unit with 240-V lines in a lab or hospital.
|
Figure 1. Example of external markings on a laser photocoagulator. Photos courtesy of Alcon Research (Fort Worth, TX) (click to enlarge). |
Safety-critical medical device marking and labeling helps to ensure the safety and effectiveness of a device. It also assists in the efficient use of the device. Product tooling, typically a long-lead-time item and often expensive to modify, may require internal or external marking. Product instruction manuals, defined as accompanying documents in IEC 60601-1, also require markings such as warning explanations and explanations of symbols used on a product. It is essential for a manufacturer to establish the target markets for a new device and investigate the medical device marking and labeling requirements for standards certification and regulatory approval. Early translation for global use is critical. Not only would it be costly to redo tooling or manuals to modify labeling, a device's market introduction could be substantially delayed.
|
Table I. Symbol standards (click to enlarge). |
In a global marketplace, harmonization of graphic symbols eases the language barriers and lessens the potential for device misuse. A review of IEC 60601-1's global regulatory importance, "A Primer for IEC 60601-1," was presented in an earlier article on MD+DI, and the differences between national standards were discussed in "National Deviations to IEC 60601-1."2,3 This article investigates the marking and labeling requirements of IEC 60601-1, including an overview of some critical symbol standards. Medical device marking and labeling to IEC 60601-1 is not complete without also considering the labeling requirements of national regulations for a targeted market.
External markings, internal markings, control markings, accompanying documents (i.e., instructions for use and service manuals), and symbols are designated requirements in the IEC 60601-1 standard. IEC 60601-1 uses the terms identification, marking, and accompanying documents to denote written text, labels, and symbols. Symbols are graphic icons used on medical devices as warnings; product classifications; or mechanical, environmental, or packaging indicators, among other purposes.
Medical Device Marking and Labeling: Symbols
For internal and external markings, IEC 60601-1 requires the use of standard symbols specified in Appendix D and in IEC 60417-1, "Graphical Symbols for Use on Equipment."4 For marking of the controls, the standard specifies conformance to IEC 60878, "Graphical Symbols for Electrical Equipment in Medical Practice."5
Symbols are often used on equipment in preference to words to save space and to preclude language differences, allowing easier comprehension. Care must be taken to ensure that the symbols are well understood and conform to the standards. Table I lists some standards that can assist manufacturers in identifying or creating appropriate symbols.
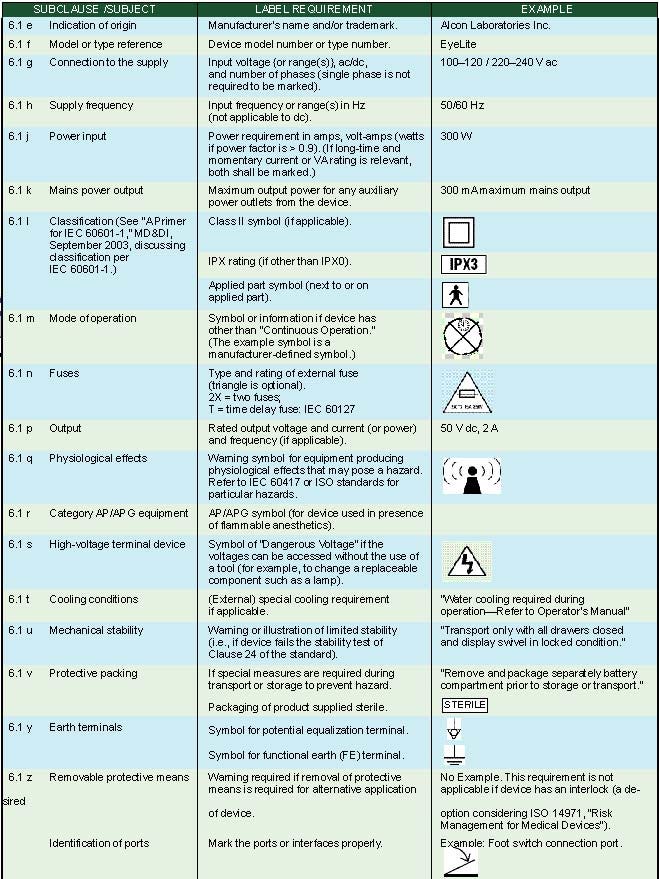
External Markings. Subclause 6.1 of IEC 60601-1 specifies the use of external markings on the outside of a medical device. Table II summarizes the information required for medical electrical products. Figure 1 illustrates some of the external markings listed in Table II using an Alcon laser photocoagulator as an example medical device.
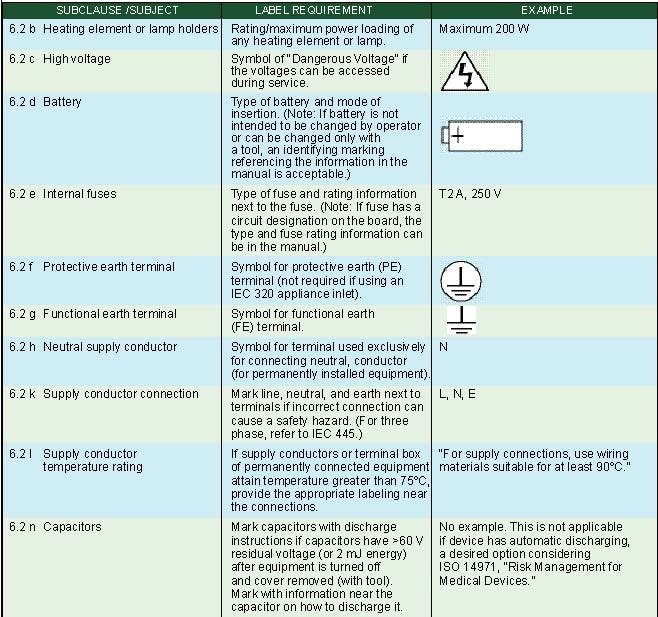
Internal Markings. Subclause 6.2 of IEC 60601-1 specifies markings on the inside of equipment or equipment parts as summarized in Table III.
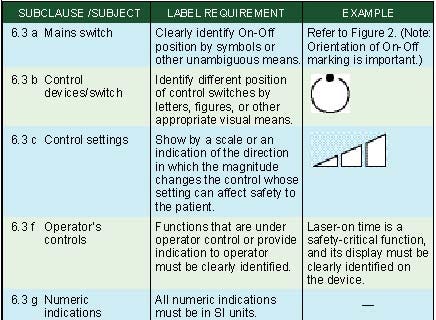
Marking of Controls. Clause 6.3 of IEC 60601-1 specifies requirements for the marking of controls and instruments. The requirements are summarized in Table IV.
SI Units
IEC 60601-1 requires all numeric indications of parameters to be in SI (Système Internationale D'Unités, metric system) units according to ISO 1000, but provides an exemption for using certain non-SI units. For example, plane angle units such as revolution, grade, and degree are exempted. Refer to subclause 6.3 g for details.
In the European Union (EU), Directive 80/181/EEC, "Units of Measurement," required metric-only labeling in the EU effective January 1, 2000; however, this date has been delayed until January 1, 2010.6 This directive is expected to supersede the IEC 60601-1 exemption. Since September 30, 1997, Japan has required that products display all numeric indications in SI units. As of February 6, 2004, UL 60601-1 began requiring SI units as stated in IEC 60601-1.7
In addition to the requirements for external, internal, and control markings, IEC 60601-1 specifies further requirements related to labeling. These include the following:
Durability of markings.
Accompanying documents.
Instructions for use.
Cleaning, sterilization, and
Maintenance instructions.
Environmental information.
Technical description.
Replacement parts information and circuit diagrams.
Transportation and storage
Information.
Medical Device Marking and Labeling: Durability
IEC 60601-1 requires that both internal and external markings be clearly legible and that all required external markings be durable. IEC 60601-1 specifies rubbing external markings with water, methylated spirit, and isopropyl alcohol. Using a cloth rag, each substance is to be rubbed on the external marking for 15 seconds. The test requirements include verifying that the external markings are clearly legible and that labels do not fall off or curl after the rub treatments.
Medical Device Marking and Labeling: Accompanying Documents
The requirements for accompanying documents are specified in subclause 6.8 of IEC 60601-1. At a minimum, accompanying documents must contain instructions for use, a technical description of the product, and an address where the user can get more information.
All markings or abbreviations on the product (including warning statements or symbols), as well as the device classification (per IEC 60601-1), must be included in the instructions for use (and also in the technical description, if provided separately).
Medical Device Marking and Labeling: Instructions for Use
|
Table II. External markings (click to enlarge). |
IEC 60601-1 treats the operator's manual (accompanying document) as a component of the equipment. The standard provides specific requirements for operator's manuals.
An operator's manual must state the function and intended application of the device. It must also contain all information necessary to operate the device safely and according to its intended function. Manuals must include information on the ports (connectors), the recognized accessories (or detachable parts) that can be connected, and any parts and materials either consumed or that need to be removed (e.g., batteries during storage) that can affect safety.
If a device contains rechargeable batteries, the manual must contain information to ensure safe use and adequate maintenance. For devices with a battery charger or a specific power supply, information identifying the charger or power supply must be provided to ensure compliance with the requirements of the standard. If a device has an additional power source (internal battery backup), a warning must be provided in the manual referring to the necessity of periodically checking (or replacing) that power source.
Medical Device Marking and Labeling: Cleaning, Sterilization, and Maintenance Instructions
|
Table III. Internal markings (click to enlarge). |
An operator's manual is required to provide information on cleaning, preventive inspection, and maintenance to be performed by the user. In addition, the frequency of such maintenance must be specified. Manuals must provide complete instructions to ensure that routine maintenance can be performed safely.
A manual must also identify those parts for which a second-party preventive inspection and maintenance is required. Manuals must provide the period of maintenance, but not necessarily the details about how to perform the maintenance. Most manufacturers of complex electromedical equipment recommend the option of having maintenance performed by the manufacturer.
For parts of equipment that come in contact with patients, manuals must describe the cleaning, disinfection, or sterilization methods that may be used. As necessary, information on sterilization must identify suitable sterilization agents; a list of temperature, pressure, and humidity requirements; and time limits that the equipment can tolerate the sterilization method. A manual must also include the limits to which the equipment was tested during the IEC 60601-1 tests.
Medical Device Marking and Labeling: Environmental Information
|
Table IV. Marking of controls (click to enlarge). |
An operator's manual must identify any risks associated with the disposal of waste products or residues generated during use. It must also provide advice on minimizing associated risks.
IEC 60601-1 also requires identifying risks associated with the disposal of the equipment and its accessories (at the end of useful life). In Europe, these requirements are expected to expand due to the Waste Electrical & Electronic Equipment (WEEE) Directive (2002/ 96/EC).8 This directive is aimed at reducing waste of electrical and electronic equipment and promoting reuse and recycling of the equipment by establishing the target of reuse and recycling as a percentage of weight. Recovery and reuse and recycling limits for medical products will be established by December 31, 2008. Currently, the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment (RoHS) Directive (2002/95/EC) does not require the medical industry to follow this requirement.9 However, the European Commission may revisit this exception by February 13, 2005. The RoHS Directive states that it ". . . shall ensure that, from 1 July 2006, new electrical and electronic equipment put on the market does not contain lead, mercury, cadmium, hexavalent chromium, polybrominated biphenyls (PBB), or polybrominated diphenyl ethers (PBDE)."
Medical Device Marking and Labeling: Technical Description
|
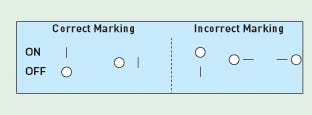
Figure 2. Orientation of power ON-OFF switch (see Table IV for label requirement) (click to enlarge). |
IEC 60601-1 requires a manufacturer to provide users with a technical description of the device (typically as part of the operator's manual or service manual). The technical description must provide all data essential for safe installation and operation of the device. The description must also include all characteristics of the device including ranges, accuracy, and precision of each indication (or display).
Medical Device Marking and Labeling: Replacement Parts Information and Circuit Diagrams
The technical description provided by the manufacturer must include instructions for replacement of interchangeable and detachable parts that can deteriorate with use.
Manufacturers must also provide or include a statement that it will make available upon request items such as circuit diagrams, component parts list, and calibration instructions to a qualified technical person to repair parts identified as repairable.
Medical Device Marking and Labeling: Transport and Storage Information
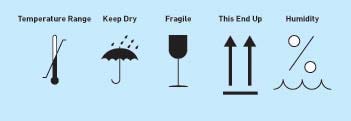
IEC 60601-1 requires the manufacturer to provide (along with the technical description of the device) the specifications for transport and storage conditions. These conditions must also be marked on equipment packaging. Typically, icons from ISO 780, "Packaging--Pictorial Marking for Handling of Goods," are used on shipping cartons to meet these transport requirements.10 It is also important to note that additional markings such as humidity conditions are often used from ISO 7000 (see Figure 3).11
Medical Device Marking and Labeling: Collateral and Particular Standards
The IEC 60601 collateral standards (i.e., IEC 60601-1-X) may call for labeling requirements in addition to the ones required for IEC 60601-1. IEC 60601-1 requires that information be provided to the user about potential electromagnetic interference; IEC 60601-1-2, 2nd ed., "Collateral Standard for Electromagnetic Compatibility," provides the details.12 Among other marking and labeling requirements, this standard requires a table covering each electromagnetic phenomenon. In addition, the IEC 60601 particular standards (i.e., IEC 60601-2-X) may delete, replace, or add labeling requirements to the ones required under IEC 60601-1. For example, IEC 60601-2-22, a particular safety standard for lasers, requires a laser burst symbol (see Figure 4).13
A review of the collateral and particular standards that apply to a medical device is a prudent investment of time and resources. The current list of collateral and particular standards published to IEC 60601-1 is available on-line at www.eisnersafety.com/recent_iec_601-1_standards.htm.
A World View of Medical Device Marking and Labeling Requirements
|
Table V. Medical Devices Directive labeling requirements compared with IEC 60601-1 requirements (continued on page 131) (click to enlarge). |
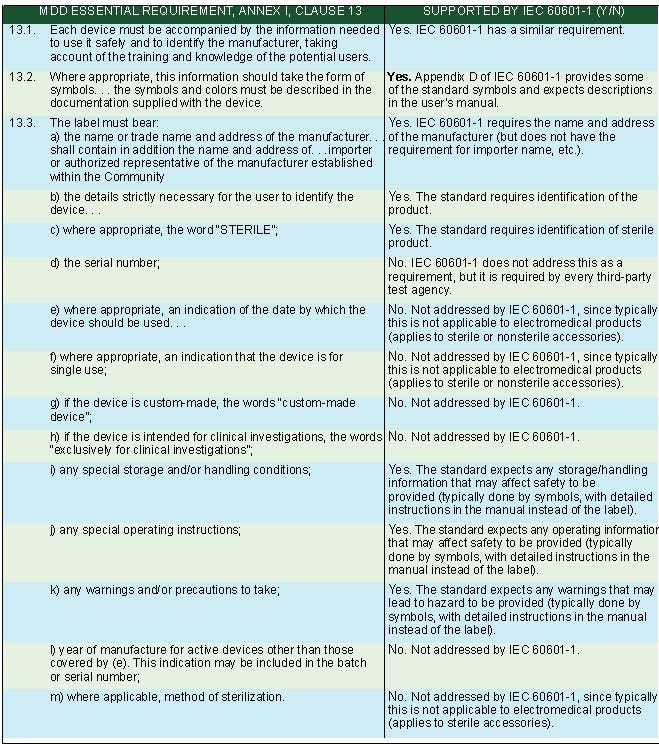
IEC 60601-1 identification and marking requirements support many of the requirements of national regulations for medical device marking and labeling. Table V provides an overview of how IEC 60601-1 supports the labeling requirements for the EU Medical Devices Directive (MDD) in the essential requirements, Annex 1, Clause 13.14 These labeling requirements are similar to the requirements in Canadian and Australian medical device regulations.
To ensure that devices meet national marking and labeling requirements, manufacturers must refer to national regulations over and above IEC 60601-1. National regulations often differ from country to country. In addition, some product types have different labeling requirements in some countries (e.g., in the United States, 21 CFR 1040 contains specific medical device marking and labeling requirements for laser products).15
Review actual medical device marking and labeling requirements closely. The recommendation to review the appropriate standards and all of the national deviations that apply to a device can't be reiterated enough. The information in this article covers common issues that apply to most medical device manufacturers. However, the standards contain much more information, and it is critical that manufacturers review them completely.
Medical Device Marking and Labeling: FDA
|
Table Vb. Medical Devices Directive labeling requirements compared with IEC 60601-1 requirements (continued from page 130) (click to enlarge). |
Similar to Europe's MDD, FDA also has regulations that pertain specifically to medical device labeling. For example, 21 CFR 801 consists of 28 regulations for medical devices.16 Not all pertain to medical electrical equipment. Search the FDA Center for Devices and Radiological Health (CDRH) Web site at www.fda.gov/cdrh for specific labeling information from the 21 CFR 801 regulations.
Medical Device Marking and Labeling: Translations
Developing a product's manual in English only may complete the manufacturer's requirements for IEC 60601-1, but not for the national regulations. For example, under Article 4 of the MDD, each member state may require product labeling to be in its national languages. Some countries allow the manual in English if the device is being used only by healthcare professionals.
The expansion of the EU in 2004 with 10 countries brings additional languages.The transposition of the MDD into national regulations is still in process in these 10 countries.
Conclusion
|
Figure 3. Icons from ISO 780, "Packaging--Pictorial Marking for Handling of Goods," and ISO 7000, "Graphical Symbols for Use On Equipment," are used on shipping cartons to meet the specifications for transport and storage conditions (click to enlarge). |
IEC 60601-1 treats labeling as a critical component of the device and provides comprehensive requirements for marking and labeling. The standard requires all information for safe installation, use, storage, servicing, and maintenance of the device to be provided to the user. For safety-significant items and for effective use of the device, the standard requires that markings be placed directly on the device. Symbols are often preferred over wording to meet such marking requirements.
Many of the requirements for IEC 60601-1 also support compliance to various national regulations for medical devices. However, defining a medical device's target market prior to deciding the marking and labeling program saves medical manufacturers both time and money. Once the target market is established, it is much easier to identify the proper national regulations that apply in addition to IEC 60601-1 tests. Then a test suite can be defined, and labeling can be selected that incorporates all requirements.
Online Resources
|
Figure 4. Laser symbol. |
http://europa.eu.int/comm/enterprise/medical_devices/index.htm
http://www.iec.ch
http://www.iso.ch
http://www.eisnersafety.com
http://www.fda.gov/cdrh/devadvice
http://www.devicelink.com
http://www.quadtech.com/resources/medical
References
1. IEC 60601-1, "Medical Electrical Equipment--Part 1: General Requirements for Safety and Essential Performance," (Geneva: International Electrotechnical Commission, 1995).
2. Leonard Eisner, Robert M Brown, and Dan Modi, "A Primer for IEC 60601-1," MD&DI 25, no. 9 (2003): 48-58.
3. Leonard Eisner, Robert M Brown, and Dan Modi, "National Deviations to IEC 60601-1," MD&DI 26, no. 2 (2004): 48-59.
4. IEC 60417-1, "Graphical Symbols for Use on Equipment," (Geneva: International Electrotechnical Commission, 2002.
5. IEC 60878, "Graphical Symbols for Electrical Equipment in Medical Practice," (Geneva: International Electrotechnical Commission, 2003).
6. Directive 80/181/EEC, Units of Measurement, Official Journal of the European Union (February 15, 1980).
7. UL 60601-1, "Medical Electrical Equipment--Part 1: General Requirements for Safety and Essential Performance" (Northbrook, IL: Underwriters Laboratories, 2003).
8. Directive 2002/96/EC, "Waste Electrical & Electronic Equipment (WEEE) Directive," Official Journal of the European Union (January 27, 2003).
9. Directive 2002/95/EC, "Use of Certain Hazardous Substances in Electrical and Electronic Equipment (RoHS) Directive," Official Journal of the European Union (January 27, 2003).
10. ISO 780, "Packaging--Pictorial Marking for Handling of Goods," (Geneva: International Organization for Standardization, 1997).
11. ISO 7000:2004, "Graphical symbols for Use on Equipment," (Geneva: International Organization for Standardization, index and synopsis, 2004).
12. IEC 60601-1-2, 2nd ed., "Collateral Standard for Electromagnetic Compatibility," (Geneva: International Electrotechnical Commission, 2001).
13. IEC 60601-2-22, "Medical Electrical Equipment--Part 2: Particular Requirements for the Safety of Diagnostic and Therapeutic Laser Equipment," (Geneva: International Electrotechnical Commission, 1995).
14. Directive 93/42/EEC, Medical Devices Directive, Official Journal of the European Union (July 12, 1993).
15. Code of Federal Regulations, 21 CFR 1040.
16. Code of Federal Regulations, 21 CFR 801.
Copyright ©2004 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like