Three Steps Toward a Successful Clinical Trial for a Combination Product
Device and drug makers can use this recommended approach to help streamline clinical trials and increase the odds of regulatory success.
November 6, 2017
The regulatory pathway for combination products is not straightforward. Multiple sets of regulations may apply to a product depending on its components (any combination of a drug, biologic, or device) and its primary mode of action. As a result, it may not be immediately obvious which path sponsors should follow to maximize their chance of receiving approval for their combination product.
FDA always treats combination products as new, even if their components have been approved previously. Combining multiple elements into a single product may create new functions or risks in addition to those of each individual component. A combination product ‘owns’ all its constituent parts’ risks: both those associated with its individual components and with their combination into a single product. Consequently, even if its components have been tested before, a sponsor must start from scratch in demonstrating the combination product’s safety and efficacy in humans.
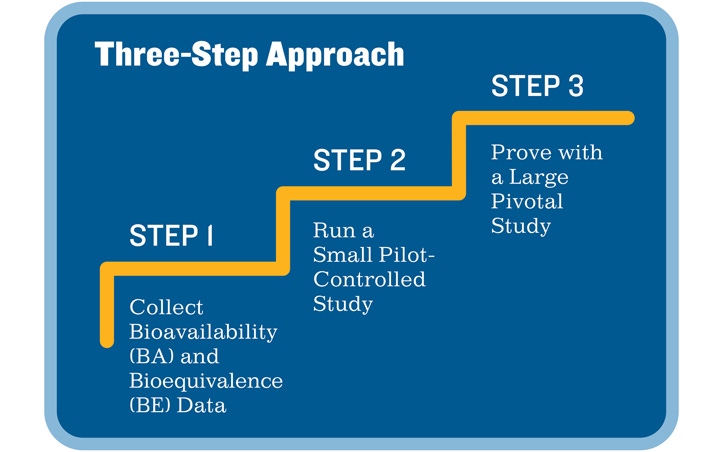
To overcome these challenges and achieve regulatory success with ease, sponsors will benefit from following a simple three-step approach as they begin their clinical trial journey. A small, 20-patient feasibility trial is a good start to establish safety, define expected adverse effects, and validate the performance of the product. The next steps, a pilot study followed by a larger pivotal trial, collect evidence of pharmacologic and device safety in a wider subject pool.
The following phased approach is particularly useful for investigational products that combine a drug or biologic with a new delivery device due to the vast difference in how a drug may act in the body when it is delivered via a new method.
1) Test Bioavailability and Bioequivalence
Sponsors should begin their clinical trials by testing the bioavailability and bioequivalence of their combination product using a randomized, double-blinded, placebo-controlled study. This study should use comparative clinical endpoints in patients with a clinical diagnosis for the indication of interest. Patients should be randomized to three groups in a 2:2:1 ratio, where the treatment arms receive the combination product, the drug or biologic alone, and the device alone (or in some cases, with a placebo), respectively. If a double-blinded design is not possible, FDA will accept an unblinded design if all trial evaluations occur in a single, blinded core facility. To demonstrate bioequivalence, both active treatments must be superior to the device alone.
2) Run a Small Pilot Study
Next, sponsors must run a prospective, two-arm, controlled study that compares the safety and efficacy of their combination product with those properties of previously cleared products. These studies should include about 50–60 patients and should be at least partially blinded to verify the safety of the combination product and the inclusion/exclusion criteria before moving to a larger pivotal trial.
3) Execute a Large Pivotal Study
Finally, sponsors must run a pivotal trial. They should base the trial size on the product’s primary mode of action, its intended use and claims, its efficacy compared with the standard of care, and the rates and identities of its adverse effects.
FDA only needs new combination products to be as good as the standard of care in terms of safety, efficacy, or both. This means sponsors may be able to use a smaller sample size. In contrast, CMS often requires a combination product to be more cost-effective and superior in safety or performance to current standards of care. Providing evidence of superiority typically requires a larger patient sample, additional data, and longer timelines, and therefore are often more expensive than non-inferiority trials.
The Role of the CRO
Combination product trials can be daunting, but this three-step approach can help streamline the development process and improve a sponsor’s ability to achieve regulatory approval. A CRO that has worked with combination products and has experience in medical devices, drugs, and biologics is well suited to assist sponsors in navigating combination product trials. The CRO can provide direction and share best practices for designing and executing these studies in a way that should yield both immediate and long-term clinical trial success.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
