Super Cancer-Fighting Nanoparticle Built like a Lego Set
April 15, 2014
MIT chemists appear to be upping the game when it comes to cancer-fighting nanoparticles, essentially creating a chemical version of Lego that can deliver three cancer drugs at a time.
The advance, which MIT announced Tuesday, could allow for multiple chemotherapeutic agents delivered in nanoparticles that reduce side effects of conventional chemotherapy by targeting the drugs directly to the tumors.
The triple-threat nanoparticles killed ovarian cancer cells more effectively than particles carrying only one or two drugs; researchers have begun testing the particles against tumors in animals, according to a paper on the nanoparticles recently published in the Journal of the American Chemical Society.
Perhaps just as importantly, the MIT chemists hit upon what seems to be a more sophisticated way for delivering the drugs in the particles--basically engineering the drugs into chemical building blocks that can then be constructed into even greater structures. (It's kind of a chemical Lego set for fighting cancer.)
"We think it's the first example of a nanoparticle that carries a precise ratio of three drugs and can release those drugs in response to three distinct triggering mechanisms," says Jeremiah Johnson, an assistant professor of chemistry at MIT and the senior author of the JACS paper.
|
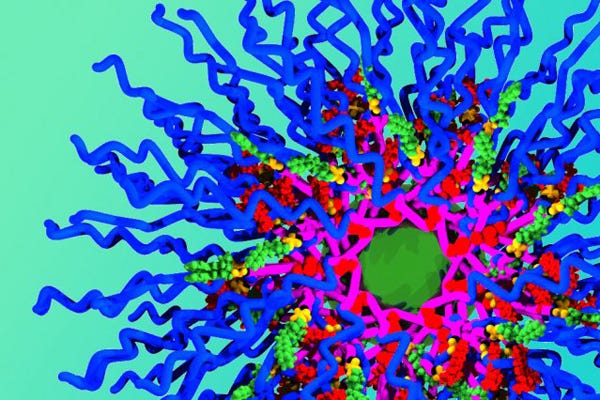
The new MIT nanoparticles consist of polymer chains (blue) and three different drug molecules--doxorubicin is red, the small green particles are camptothecin, and the larger green core contains cisplatin. (Image courtesy of Jeremiah Johnson) |
Johnson and colleagues say they overcame the inherent limitations of the two methods most often used to produce drug-delivering nanoparticles.
In the past, such particles have involved either encapsulating small drug molecules inside the particles or chemically attaching them to the particle. Such methods become increasingly difficult as each new drug is added, and using both methods at once has limitations, according to MIT.
So instead of building the particle and then attaching drug molecules, Johnson created chemical building blocks that already include a drug--joining them together into a specific structure.
Each building block includes three components: the drug molecule, a linking unit that can connect to other blocks, and a chain of polyethylene glycol (PEG), which helps protect the particle from being broken down in the body.
With such building blocks, Johnson and colleagues could then essentially play a chemical version of Lego (Johnson calls it "brush first polymerization."), linking hundreds of the building blocks together.
Johnson developed, called "brush first polymerization."
"This is a new way to build the particles from the beginning," Johnson says. "If I want a particle with five drugs, I just take the five building blocks I want and have those assemble into a particle. In principle, there's no limitation on how many drugs you can add, and the ratio of drugs carried by the particles just depends on how they are mixed together in the beginning."
The MIT researchers could then assemble their building blocks in a highly strategic fashion to attack ovarian cancer:
"For this paper, the researchers created particles that carry the drugs cisplatin, doxorubicin, and camptothecin, which are often used alone or in combination to treat ovarian cancer. Each particle carries the three drugs in a specific ratio that matches the maximum tolerated dose of each drug, and each drug has its own release mechanism. Cisplatin is freed as soon as the particle enters a cell, as the bonds holding it to the particle break down on exposure to glutathione, an antioxidant present in cells. Camptothecin is also released quickly when it encounters cellular enzymes called esterases. The third drug, doxorubicin, was designed so that it would be released only when ultraviolet light shines on the particle. Once all three drugs are released, all that is left behind is PEG, which is easily biodegradable."
Johnson's lab is now experimenting with particles that carry four drugs.
Says Johnson: "It's important to be able to rapidly and efficiently make particles with different ratios of multiple drugs, so that you can test them for their activity," he says. "We can't just make one particle, we need to be able to make different ratios, which our method can easily do."
Refresh your medical device industry knowledge at MD&M East, June 9-12, 2014 in New York City. |
There have been a number of efforts to attack cancer cells using nanotechnology. Recently, for instance, Korean scientists inserted tiny robots within genetically modified salmonella bacteria that is attracted to chemicals produced by cancer cells including vascular endothelial growth factor. The microscopic robots, which measure 1 ?m in width, contain capsules with cancer-fighting drugs.
Cornell University researchers have used tiny gold-based particles that could target cancer cells and later be heated up to kill them. MIT engineers also devised nanoparticles to ferry doxorubicin into cancer cells while also delivering gene-therapy agents. Last year, researchers from Harvard developed cancer-fighting nanobots approximately the the size of a virus using protein and DNA strands. The bots are cylindrically shaped, enabling them to be stuffed with molecules of cancer-fighting drugs.
Chris Newmarker is senior editor of MPMN and Qmed. Follow him on Twitter at @newmarker.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
