How Consumer Technology Is Driving Innovation in Medical Devices
With lower costs, better form factors, and new features, wearable biometric sensor technology is now enabling ambulatory mobile healthcare, with a focus on prevention, screening, and disease management.

Even in the midst of turbulent market dynamics, wearable devices have continued to see a tremendous amount of growth around the world. While key market players have seen their respective market shares increase or decrease, the macro-level growth remains strong--IDC reports over 100M units sold in 2016, up 25% over 2015.
Taking a slightly longer term view, the technology in these devices has also seen a tremendous amount of innovation in the last 20 years. It wasn't that long ago that ECG chest straps were cutting edge technology before there was such a thing as "wearables." Since then we've seen GPS, accelerometers, gyroscopes, optical biometric sensors, galvanic skin response sensors, and much more integrated into wearable devices of all kinds.
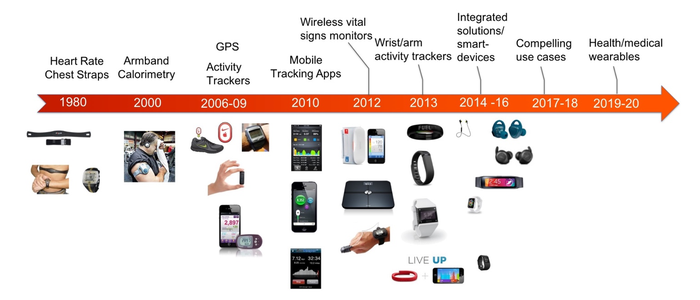
These technologies have enabled new user experiences, primarily in activity and fitness tracking where the wearables market has grown up. However, new advancements in wearable technology, particularly biometric sensor systems, have opened up new possibilities for wearables in healthcare and medical devices. In fact, Frost & Sullivan expects the market for clinical-grade medical wearables "dedicated to chronic disease monitoring and other clinical applications" to reach $18.9B by 2020 at a compound annual growth rate of 29.9%.
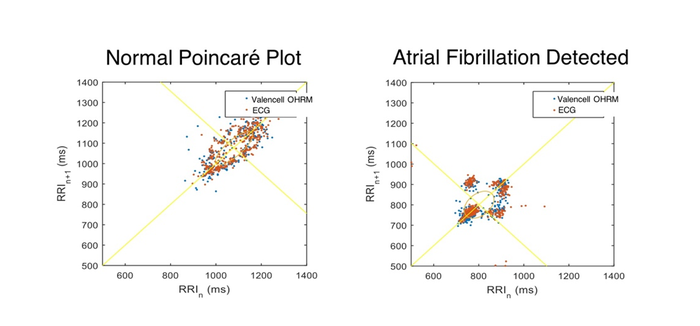
This has become a reality because wearable optical sensor systems are now demonstrating performance levels suitable for medical use cases. For example, biometric sensor systems embedded in consumer wearable devices have been shown to detect the presence of atrial fibrillation. This is done by measuring and analyzing R-R intervals, the time between beats of the heart, which display different patterns for healthy and non-healthy hearts.
The chart below on the left is a healthy heart displaying a "torpedo" shape from lower left to upper right, while the chart on the right shows clusters in the bottom left of the chart, a clear indication of atrial fibrillation. The data in these charts was collected from a consumer audio earbud with advanced biometric sensor technology to measure heart rate and R-R interval. A key significance of ear-based heart rhythm monitoring is that it can be achieved very accurately (due to high perfusion and low motion artifacts associated with the ear location) and can be truly mobile (embedded in ear-worn devices that people use on a regular basis).
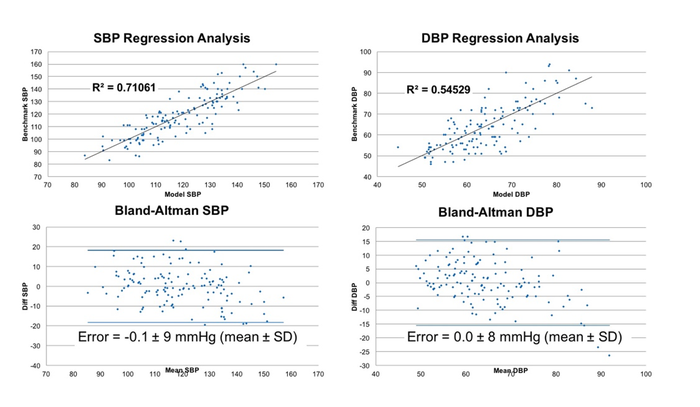
Another example is in blood pressure. Advanced optical biometric sensors similar to those found in consumer wearables and hearables have demonstrated the ability to estimate blood pressure on par with a consumer-grade blood pressure cuff. Valencell is conducting ongoing research in which we've collected hundreds of data sets on more than 100 different study participants comparing an optical biometric sensor system to the blood pressure cuff and the results are compelling.
Advancements like these and others are leading many in the industry to say that innovation in consumer biometric wearables is beginning to outpace that of medical monitoring technology--not only in capability but also in terms of cost. With lower costs, better form factors, and new features, wearable biometric sensor technology is now enabling ambulatory mobile healthcare, with a focus on prevention, screening, and disease management.
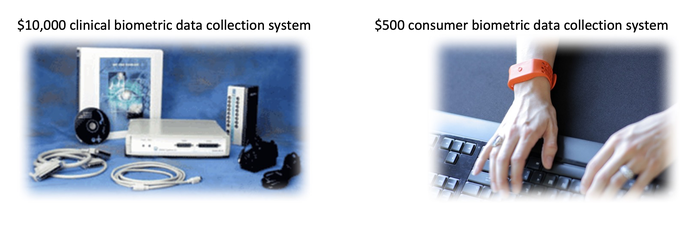
Take these two systems: On the left is a computer-based biometric acquisition system for life science research and clinical education. On the right is a biometric data collection system for building consumer wearables. The one on the right can do nearly everything that the one on the left can do for a fraction of the cost and in a much more convenient form factor.
This does not mean that consumer wearables on the market today are ready for medical use today, but rather that the technology is ready for medical validation studies. To make the move from consumer to medical wearables you will almost certainly see partnerships and/or acquisitions between consumer wearables and medical devices for at least these two reasons:
Consumer wearables companies know how to make devices that people want to wear regularly, but they lack the infrastructure, processes, and experience required for medical grade accuracy, clinical validation, and regulatory approvals.
While medical device companies have the right infrastructure, processes, and experience for medical devices, they lack the knowledge of how to make devices that consumers actually want to wear on a regular basis.
However, these two markets are structured very differently, making collaboration challenging:
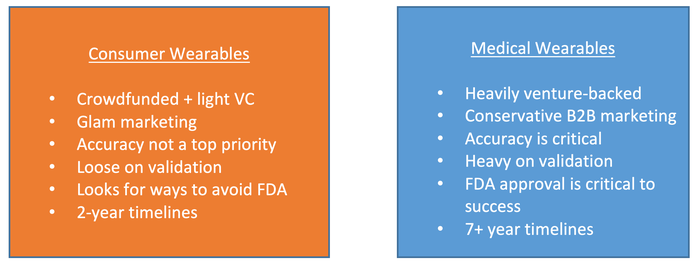
Despite those challenges, the opportunities in the market are huge, as mentioned above, so we are starting to see market pull for advanced biometric sensors in medical wearables in several different categories.
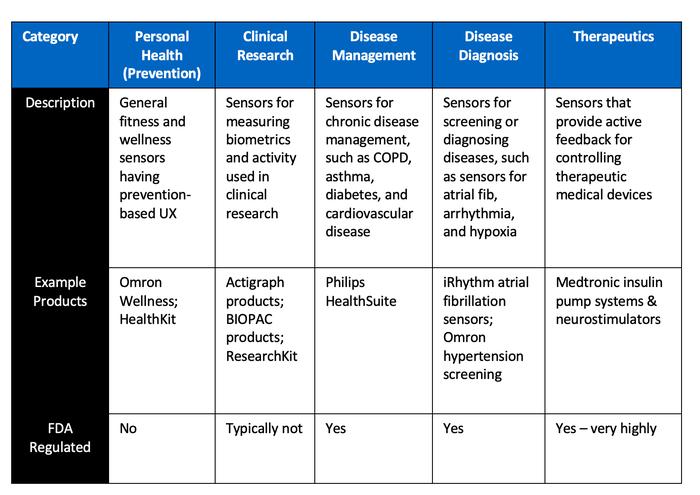
One particular point of friction is merging the rather chaotic exploration and rapid innovation of consumer wearables with the methodical discipline of clinical validation. In particular, FDA regulation is always one of the first questions asked about any form of consumer medical device. However, the FDA approval process is not the bottleneck in this case.
Use case validation is the key obstacle and here's why: The steps to commercialize one of these devices will typically look like this:
Device prototyping: 2-5 weeks
Feasibility testing in the lab: 1-2 weeks
Use case validation in the field: More than a year
Independent clinical validation: More than 6 months
FDA approval (510K): 3-9 months
All the research, testing, and validation leading up to the FDA approval process are the largest time and resource commitments in this process. This is further complicated by the fact that most medical device and consumer wearables companies do not currently have in-house capabilities to perform use case validation. So these companies will either need to acquire or outsource this critical step in the process.
We are still in the very early stages of the consumer medical wearables product and market development, but it's already clear that success in this market with require both an understanding of the consumer market and clinical validation. Companies that can apply medical-style validation to compelling consumer medical use cases using innovative wearable sensor technologies are best poised to find success in the marketplace.
Steven LeBoeuf, PhD, is the president of Valencell, a Raleigh, NC-based company focused on innovating biometric sensor technology.
[Images and charts courtesy of VALENCELL INC.]
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
