Boston Sci, Mayo Clinic Reveal Major Innovation Partnership
March 17, 2016
Boston Scientific and Mayo Clinic, which each have a major presence in Minnesota, have just revealed a multi-year partnership that could yield a dozen new or revised devices, with four clinical trials possible this year alone.
Nancy Crotti
|
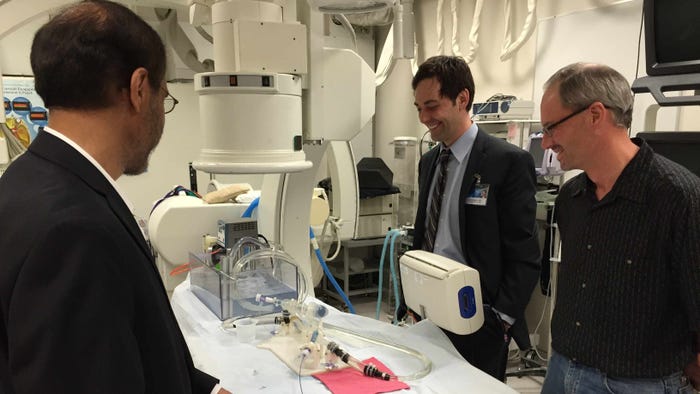
Daniel B. Spoon, M.D. from the Mayo Clinic (center) evaluates the performance of a centering catheter on a benchtop model along with Tim Ostroot (right) from Boston Scientific as Gurpreet S. Sandhu, MD from the Mayo Clinic. Image from the Mayo Clinic. |
Location, location, location - it's not just for real estate.
Over the past three years, Mayo physicians and researchers have teamed with BostonSci engineers to discuss roughly 50 ideas for devices that could improve patient care, under an agreement that protects the intellectual property on both sides, according to officials from both organizations.
The first idea up for trial came from Dr. Gurpreet Sandhu, an interventional cardiologist and director of Mayo Clinic's cardiac catheterization lab. It's a catheter whose end opens into a funnel. The funnel centers the catheter, making it easier, faster and less dangerous to thread a wire through a calcified artery against strong blood flow from the heart. The wire enables placement of an artificial aortic valve.
That procedure currently takes 2 to 25 minutes, depending upon the calcification, scarring, and the strength of the blood flow through the narrowed opening, Sandhu said. Risks to patients include longer exposure to X-rays and time under anesthesia, damage to the artery and valve, and the possibility of stroke if part of the calcium should break off.
"I have been doing these procedures for a while, and this was always a concern," said Sandhu, who conceived the funnel idea. "At Mayo, we are constantly trying to develop new technologies and safer ways for treating our patients."
Direct collaboration among physicians and engineers significantly speeds the technology development process, he added.
"The advantage was, since Boston Scientific has a large engineering presence in Minnesota, in Minneapolis, it's been very easy for the engineers to come down to Rochester to interact with us," Sandhu said. "The location has made a big difference."
Rochester is about 1-1/2 hours from the Twin Cities by car.
Location wasn't the only advantage. The repeal of the federal medical device tax freed Boston Sci to devote more money to device research, according to Doug Pennington, the company program lead for the Mayo collaboration.
Two to three Boston Sci employees, mostly engineers, work with Mayo staff to put a prospective device on a clinical, regulatory and marketing path, Pennington explained. In addition to interventional cardiology, those devices would come from areas that BostonSci develops in Minnesota, including heart rhythm management, endoscopy, neuromodulation, urology and pelvic health.
"What we end up doing is acting like a startup within a larger organization with all of the resources that a big company has to offer," Pennington said. "It's a multi-million-dollar project."
Collaborators are working on using Boston Sci's Precision Spectra Spinal Cord Stimulator to control blood pressure and blood vessel resistance in heart failure patients during exercise by intercepting messages of muscle fatigue and shortness of breath.
The device tax repeal helped replace venture capital that has flowed away from medtech due to frustration with long regulatory pathways and even longer waits for returns on investment, said Jim Rogers, chair of Mayo Clinic Ventures. The in-house not-for-profit has a product development team, but not the high-end design capabilities that a medtech giant can bring to the task of getting an idea to market, Sandhu added.
The legal agreement that enabled this collaboration is hard to reach, but after three years, considerable trust has grown on both sides, Rogers said.
"We have this ability to kind of look at the best available kind of solution, and it may come originally from them or originally from us, but we work together to make something that we think can benefit patients," he said.
The collaboration is not the first for Mayo, which previously worked with Roche Diagnostics to develop a real-time PCR platform. But it "has become a bit of a magnet" for other companies interested in working together similarly with Mayo, according to Rogers.
"We're certainly open to it," he said. "If someone else is willing to work with us, we're certainly willing to talk with them."
"Getting potentially four products into first human use is, we think, a good mark of the success of the collaboration," Pennington said. "Mayo has a certain kind of doctor who is not only interested in practicing medicine but actually impacting it and changing it and improving it... Finding that common overlap is not an easy endeavor, but once it's done the incredible resources that both have are able to synergistically interact and it's a wonderful thing. It just takes a lot of time and a lot of presence on both sides and a lot of commitment to make it happen."
FDA approved the Precision stimulator in 2004 to transmit electrical signals to the spinal cord to decrease chronic pain in the body, arms and legs.
"We are slowly starting the process of one device at a time," Sandhu said.
Learn more about cutting-edge medical devices at BIOMEDevice Boston, April 13-14, 2016. |
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like