A New ‘Force’ in Injectable Drug Delivery
April 25, 2017
A new approach to delivering injections could ease administration and open up design possibilities.
Daphne Allen
Developing delivery devices for injectable drugs has never been as challenging as it is today. Thanks to changing healthcare models, a growing number of patients are being asked to self-administer therapies for cancer, arthritis, multiple sclerosis, and other chronic illnesses. But patients aren't professionals. "Patients who will need to take injections are not familiar with using a syringe and needle," Steven Kaufman, Global Business Development Lead for Bespak, told attendees at Pharmapack Europe. "They will need an easy-to-use and intuitive self-injection device." His presentation on February 1 was titled, "What innovations and best practices are helping to support patient compliance in the area of self-injection devices?"
|
Bespak is innovating the delivery of difficult-to-administer formulations, such as biologics, with its VapourSoft compact energy source. |
As designers set out to develop such devices, they are encountering another challenge--changing injectable drug formulations. "Many new injectables are as gloopy and viscous as honey," Kaufman said. And with higher volumes making up many of these newer drug doses--sometimes up to 10 ml, Kaufman later told Qmed--"Viscosity can cause serious issues with completeness of injections. Patients have difficulty holding a device against their skin for very long."
The goal, then, is to develop devices that allow patients to self-inject easily and quickly. But because injection devices have typically utilized some sort of spring-loaded force to move the plunger during injection, the higher viscosity levels require higher forces, which has introduced yet another challenge: breakage of glass cartridges under higher forces. "Pharma companies are also quite concerned about the force required to deliver a complete injection and injection time," he adds.
Bespak decided to look for alternatives to spring-loaded force. With extensive in-house experience producing canisters for metered-dose inhalers, the company began exploring the propellants typically used for such technology. "We took the asthma-drug canister and shrunk it down to the size of a nickel, and then used liquefied gas to deliver injectables, so the device isn't under load." Kaufman says. VapourSoft was born.
Kaufman described the solution at Pharmapack and later spoke to Qmed. "It consists of a small canister placed at the back of an autoinjector, but unlike with conventional spring autoinjectors, the device is not under load," he says. Upon activation, "the canister opens and releases the liquefied gas to deliver the dose."
The drug is still held in its original primary container, he adds, so there's no change in drug-contact materials. "Our VapourSoft canister is just affixed to the back, with or without a plunger rod. And we can use the same propellant as used for asthma therapies, HFA, and other gases. It is just used here as a power source to push against the plunger or stopper."
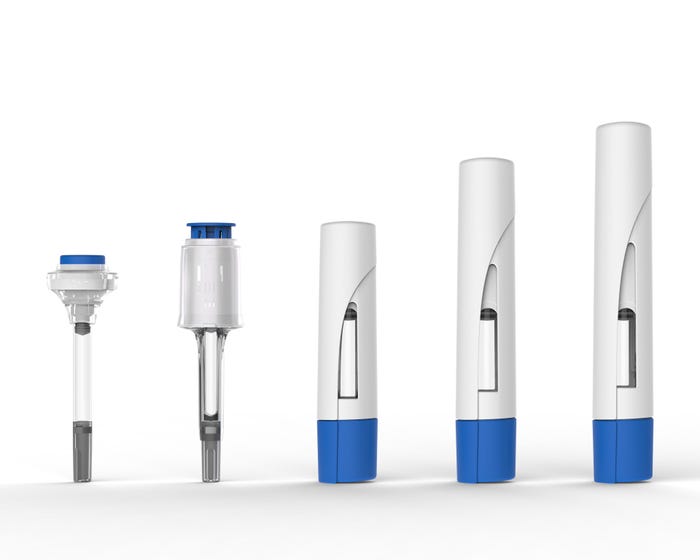
Bespak can incorporate VapourSoft technology into traditional pen-shaped autoinjectors. "We can also make some of the smallest available autoinjectors," Kaufman says. "Our Syrina 2.25 is one of the smallest 2.25-ml autoinjectors on the market. We can even adapt the same device to also handle a 1 ml PFS. The small footprint of VapourSoft allows you control the space required to make the device."
|
Bespak's Syrina range of assisted syringes and auto-injectors, utilising its VapourSoft power source. |
And the standard VapourSoft can is able to deliver volumes up to 10 ml, which could be required for wearable devices, Kaufman said. "The tipping point is the viscosity. VapourSoft can quite easily deliver standard injection volumes in less than 10 seconds with the right needle for auto injectors, and we can control injections of larger volumes over a period of time for wearable devices."
Unique shapes are even possible, he adds. "Using VapourSoft means that the device does not need to be in a linear pen-like format. A number of shapes and sizes are now available when companies customize their device."
Bespak is still very much invested in traditional spring-loaded autoinjectors. "We can use different technologies for different challenges," Kaufman says. "Springs are very successful and can be innovative. UCB recently launched its Cimzia Autoclicks autoinjector, which is based on Bespak's ASI spring powered technology."
"Device companies have to think about how to differentiate themselves and give pharma companies more tools," he adds.
And that also includes tools to help encourage patient compliance. "Patient fatigue can happen when patients need to hold a device to skin up to 10 to 15 seconds," he says. "That is inconvenient, and patients could stop an injection early and then get an incomplete dose. And then patients might go off therapy.
"Pharma and device companies need to do more," he continues. "They need to develop devices that are more flexible for patient populations, with a different form factor and that address injection time."
Kaufman believes that VapourSoft could help with compliance by easing self-administration. "With VapourSoft, patients don't need to be overly aware of function--they just need to hold the device to their skin and activate it," he says.
|
Bespak's Syrina S model is a compact auto-injector designed to deliver up to 2 ml of viscous drug formulations. |
Bespak has conducted user studies for VapourSoft-driven autoinjectors and designs, and to date end-users have reported feeling comfortable with the new delivery system, Kaufman reports.
"Self-injection devices can help to improve patient compliance for those taking or giving injectable medications," he concluded during in his presentation at Pharmapack Europe. "A true partnership between the biopharma companies and device companies working with patients is the only path that will lead to success."
Daphne Allen is executive editor of Pharmaceutical & Medical Packaging News and a contributor to Qmed. Reach her at [email protected] and on Twitter at @daphneallen
******************************
User-centered design will be one of the major themes at the upcoming Medical Design & Manufacturing (MD&M) East 2017 conference in June. Check out these sessions and more:
To Err is Human, But How Do You Prevent It? Speaker: Michael Wiklund, General Manager, Human Factors Engineering, UL LLC, Wiklund R&D
Panel: What Does Usability Mean Today for Medical Device Engineers? Moderator: Stephen Wilcox, Principal, Design Science; Panelists: Daniel Kosoy, MD, Partner, Athenian Venture Partners; Jim Lebret, MD, Physician, New York University School of Medicine; and David Brick, MD, FAAP, FACC, Clinical Associate Professor, New York University Medical Center
How Usability Research and Engineering are Changing Medical Device Development Speaker: Philip Remedios, Principal, Director of Design & Development, BlackHägen Design
New Tools for Obtaining Better Data from Contextual Inquiry Speaker: Stephen Wilcox, Principal, Design Science
About the Author(s)
You May Also Like





.png?width=300&auto=webp&quality=80&disable=upscale)
