Better Imaging for Prostate Cancer Patients?
Blue Earth Diagnostics shines at the 2018 annual meeting of the Society of Nuclear Medicine and Molecular Imaging, with fleshed out results from the LOCATE trial.
June 27, 2018
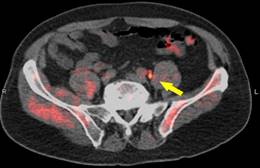
A new study is giving greater in insight on the effectiveness of imaging patients suffering from prostate cancer. Data from the LOCATE trial shows that the addition of fluorine-18-fluciclovine positron emission tomography/computed tomography (PET/CT) to the diagnostic work-up of patients with biochemical recurrence of prostate cancer locates previously undetected lesions and changes treatment management for the majority of patients.
Blue Earth Diagnostics presented data from LOCATE, which evaluated the test on Tuesday at the 2018 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging. The Burlington, MA-based company’s test was approved by FDA in 2016.
“The objective [of LOCATE] was to access the impact of positron imaging for patient management,” Peter Gardiner, Blue Earth Diagnostics CMO, told MD+DI. “These were men with recurrent prostate cancer.”
For the LOCATE trial 213 men with biochemically recurrent prostate cancer were evaluated with 18F-fluciclovine PET/CT after having negative or equivocal findings on conventional imaging, such as a bone scan, CT or MRI.
This first prospective multi-center study was conducted at 15 sites in the U.S., including both private practices and academic settings. It focused on the association between scan positivity and the clinical variables of recurrence site, practice setting, prostate-specific antigen (PSA) level, and Gleason score (system of grading prostate cancer). Questionnaires completed by treating physicians documented changes in management after the PET study. Trial results showed that 59% (126/213) of patients had their clinical management changed by findings from 18F-fluciclovine imaging.
Of those changes, 78% (98/126) were classified as major. Disease was detected in the prostate, as well as other tissue, including pelvic and abdominal lymph nodes and, less commonly, bone. Both positive and negative scans impacted patient management. No difference was seen in the rate of positive scans or in the rate of management changes between private practices and academic settings. In addition, no association was found between Gleason score at diagnosis and positive scans.
Plans call for Blue Earth Diagnostics to push for the LOCATE study results to be published.
“There is a manuscript submitted already submitted to the urology journal and it’s undergoing review at the moment,” Gardiner said. “We’re keeping our fingers crossed that will be published.”
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
