Human Factors: Moving in the Right Direction
Originally Published MDDI August 2004
August 1, 2004
|
Michael E. Wiklund is vice president in charge of the Human Factors Research and Design Group at the American Institutes for Research (Concord, MA). |
Are you familiar with the terms ergonomic and user-friendly? Do you, on a daily basis, think about whether the products you develop or use are intuitive? Chances are, you answered “yes” to both questions. This signals a substantial change in the visibility and effect of the multidisciplinary profession called human factors engineering. Twenty-five years ago, the profession had a relatively low profile in the medical domain. However, it had the potential to make significant contributions to the quality of medical devices requiring user interaction. Fortunately, it has a higher profile today, enabling it to have a positive influence on the product development process.
Background
Fundamentally, human factors engineers focus on making products safe, effective, easy to use, and appealing. With roots dating back to the early 1900s, the profession gained ground during the mid-to-late twentieth century, as companies dealt with increasingly complex electromechanical and computer-based machinery.
Still, even in the late 1970s developers and consumers focused little attention on user interaction quality as compared with other engineering and design considerations, such as reliability and visual appeal. In short, most of the user-interface design work remained a seat-of-the-pants endeavor that drew on an engineer's common sense. This was particularly true in the medical domain, where there was a presumption that any medical device would be used by trained professionals. Everyone believed that their training would make up for any design shortcomings.
|
Paul Kirley, Stuart Karten, and Dennis Schroeder examine the Clarion Speech Processor by Advanced Bionics. The device works with a cochlear implant to help people hear. |
Decades ago, if a medical device was difficult to learn to use, caregivers were more likely to blame themselves rather than the device, and they would find ways to cope. Flash forward to the present and you find the pattern has practically reversed itself.
Now, caregivers are well-acquainted with well-designed products matching their physical, intellectual, and emotional needs, partially because of products proffered by the consumer electronics and software industries. They are now more likely to call a product obstreperous than blame themselves for their ineptitude. This goes double for nurses, who normally take a can-do attitude toward arduous tasks, including some that intimidate physicians, but have little patience for ill-behaved equipment. In fact, they have been known to make emphatic statements, such as “I wouldn't want that thing on my unit,” after trying their best to use a product that seems designed by engineers for engineers.
Indeed, society as a whole and the medical community in particular have come to demand products that are engineered to be intuitive, feel right, and work nicely. And, it's about time—user-friendly medical devices are generally safer devices. Intuitive operation and lower error rates generally go hand in hand.
Raised Expectations
|
Poorly labeled controls and maintenance tags that covered |
Taking a broad view, design expectations started to shift in the early 1980s, accelerating through the 1990s. The shift occurred because of many factors, including the accident at Three Mile Island (TMI) in March 1979, which culminated in a partial core meltdown. Even though that statement seems farfetched, TMI really did influence consumer expectations about human factors engineering. It helped bring the discipline to the public's attention and created a demand for human factors engineers.
TMI accident investigators determined that simple things, like poorly labeled controls and maintenance tags covering important gauges, contributed to operational difficulties associated with the incident.1 In response to this finding, the U.S. Nuclear Regulatory Commission (NRC) directed all domestic nuclear power plant operators to conduct human factors reviews of their control rooms. These reviews identified many human factors engineering deficiencies. These led to control-room renovations that promised to improve safety through better operability.
The NRC's safety initiative created a strong demand for human factors engineers that lasted about a decade. Later, when the power plant renovations were largely completed, the demand waned. This led some human factors engineers to migrate to the medical domain because it posed some of the same analytical and design challenges, particularly controlling complex systems through a relatively simple user interface. However, human factors engineers emigrated in greater numbers to the software industry. Companies like Microsoft and Oracle built large usability engineering groups to enhance their applications.
It was also about 25 years ago that the computer industry was rolling out a new kind of product called the personal computer. The first desktop machines employing the disk operating system (DOS) were suited for use by people who knew something about programming. Those with little or no prior computer experience found the machines quite daunting. In fact, their travails were the subject of numerous cartoons depicting people at their wit's end: images of clenched fists, red faces, and steam blowing out the ears.
|
People with little or no prior computer experience found that they could use the first Apple Macintosh computer easily. |
But along came the Apple Macintosh computer—some consumers' first introduction to complex operations made simple and accessible, the automobile notwithstanding. Suddenly, novices could draw upon their basic intelligence and intuition to accomplish computer-based tasks, ranging from word processing to financial management to artwork production.
The race was on to make computers, software applications, and other aspects of high technology more user-friendly. Jump forward a quarter century and you find a marketplace in which the majority of manufacturers, including medical device manufacturers, make bold claims about their products' ease of use. In some cases, the claims have substance because they arise from a developer's investment in human factors engineering. It is a process punctuated by usability tests that prove, or disprove, that representative users can operate the targeted products.
Many medical devices seem to be getting easier to use and safer in the process. However, Julian Goldman, MD, a practicing anesthesiologist at Massachusetts General Hospital and professor at Harvard Medical School, is reserving a final judgment. The former chair of ASTM's Committee F29 on Anesthetic and Respiratory Equipment says that “things are getting better slowly—slowly—slowly.” Reflecting on his observations after almost two decades of clinical practice, he cites the lack of equipment integration as a major hurdle to greater progress. “Manufacturers continue to assess their devices in isolation,” he notes. “They are not addressing all of the environmental factors, including other equipment and different types of users, in their design development process.”
Goldman adds that “some manufacturers may face economic limitations. Good human factors practice can be time-consuming and expensive. It may be hard to make an argument in favor of a large investment when a product has a short life cycle.…Manufacturers are going to be tempted to let consumers be their beta testers, knowing they [the manufacturers] can fix things for the second release. Of course, this is not the best approach from a patient safety perspective.”
While Goldman's comments ring true, there also are more medical company CEOs than ever before who are familiar with human factors engineering—a big change from years ago. And some have made usability an important part of their brands, recognizing that customers frequently cite usability as one of their highest design priorities.
Some company leaders have learned that human factors engineering makes good economic sense, considering potential rewards like faster product development cycles and increased sales linked to customer preferences for a “user-friendly device.” Also, reduced demand on customer support and reduced liability exposure have good implications for a company. To capitalize on these rewards, firms have invested substantially in human factors. They have either set up internal specialty groups or retained human factors consultants to work with their engineering and marketing teams. For example, several large companies, including
Abbott Laboratories, Alaris Medical Systems, Ethicon Endo Surgery, GE Medical Systems, Medtronic, and Siemens Medical Solutions, either employ or engage human factors specialists who focus on ensuring each product's safety and giving it a competitive edge. Many smaller companies do this as well, although they tend to hire consultants in lieu of being able to afford a full-time specialist.
Still, some medical companies have yet to embrace human factors engineering as a key to customer satisfaction and quality care. Goldman attributes this condition to the fact that human factors investments pay off mostly in the long term. Thus, manufacturers may have an easier time marketing products that are user-friendly, but the benefits of higher workforce productivity and satisfaction, for example, accrue to their customers, such as hospitals.
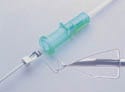
|
To prevent needle sticks, a metal fitting extends over the end of the needle of the B. Braun Medical Inc. Introcan Safety Intraveneous Catheter. |
Meanwhile, the complexity of technology seems to be on another upswing, largely due to the increasing power and decreasing cost of microprocessors. So the industry may be at a turning point. Companies must decide between spending more on human factors engineering to ensure the ease-of-use of devices, and limiting their spending, thereby triggering a decline in the caregivers' ability to cope with increasingly complex devices.
And speaking of device complexity, one has to wonder whether caregivers have already reached their limit in terms of computer-based interactions replacing good old knobs and dials. More medical devices than ever require users to navigate menus and to mouse-click. Meanwhile, recent studies indicate that a decreasing percentage of doctors know how to use a standard stethoscope effectively. These may be symptoms of the shift from a more hands-on style of equipment interaction and care to one that is mediated by computers, as well as the associated shift in medical education.2
Toward a User-Centered Design Process
With due respect to those who oppose government regulation, FDA's updated quality system regulation that calls for the application of good human factors practice in medical device development looks like progress.3 For years, human factors engineers who struggled to have an effect in various medical companies hoped for such a regulation. Regulating the application of human factors in medical device design would give them ammunition to use in the battle for influence within their engineering departments.
Historically, other engineering considerations tended to trump human factors requirements when push came to shove within engineering teams. The regulatory imperative to apply good human factors brought a welcome change in the ability of human factors engineers to make a difference. They were able to push back on technical solutions that were not user-friendly and to encourage those that were.
|
The Phillips HeartStart Home Defibrillator uses extensive voice prompts to talk people through the delivery of cardiopulmonary |
FDA's updated regulation, released in 1996, stresses the importance of identifying user needs as a precursor to user-interface design. The agency's Human Factors Program's Web site states, “Research has suggested that the frequency and consequences of medical device use errors far exceed those arising from device failures. Therefore, product developers must consider device use and use-related hazards to ensure that their devices will be safe.”4
FDA's prescribed development model encourages designers to build devices in response to demonstrable user needs rather than the desire to showcase new technology. In addition, the regulation calls for user feedback during the development process so that problems can be addressed before the product goes to market. This means that the initial customers and patients will no longer have to serve as the guinea pigs.
Prior to regulatory change, manufacturers tended to address human factors requirements in an informal, ad hoc manner. In the late 1970s, most medical device manufacturers equated human factors engineering with market research. Developers considered obtaining design feedback from preferred customers and folks visiting trade show booths and hospitality suites to be a reasonable strategy for addressing user needs in the design process. As one might expect, this approach yielded unreliable user preference and performance data, if any, and burdensome designs.
For example, a new generation of microprocessor-based devices, such as patient monitors and infusion pumps, came to market in the late 1980s. They seemed better suited for use by engineers and programmers than by computer-phobic caregivers. Consequently, caregivers spent a lot of time adjusting to the new technology, which distracted them from direct patient care. You also saw caregivers developing coping strategies, such as learning by rote to perform the basic tasks, and ignoring the rest of an instrument's advanced capabilities.
Still, Goldman regards the kind of research conducted in hospitality suites as better than no user research. He strongly advocates a formal process for collecting design input from users at several stages of development. But, he adds, “Showing mock-ups in a hospitality suite provides some amount of feedback that can improve a product.”
Today, you see exemplary companies working harder and more effectively at user-interface design. They establish human factors programs that ensure the systematic definition of user requirements (which FDA calls design inputs), followed by the rigorous application of human factors in design and iterative usability testing. The resulting design and associated performance data (which FDA calls design outputs) typically set the stage for a smooth regulatory approval process and good customer acceptance of the final product.
In addition to FDA, the Association for the Advancement of Medical Instrumentation (AAMI) warrants recognition. They have produced a continuing series of standards for the application of human factors engineering principles to the design of medical devices. AAMI published its first human factors standard in 1988, which drew extensively from military standards on human factors. Thirteen years later, the standards organization released ANSI/AAMI HE74:2001, “Human Factors Design Process for Medical Devices.” The document, which has been endorsed by FDA, describes alternative ways for companies to conduct an effective human factors program.
Notable Products
An informal survey of several human factors professionals working in the medical industry identified the following products as landmarks in terms of their human factors engineering.
Pulse Oximeter. The Nellcor N-100 pulse oximeter, dating back to the early 1980s, is a landmark product. Providing a simple and intuitive indication of oxygen saturation level through multiple sensor channels, the product proved to be a boon to patient safety. It was and remains extremely popular among clinicians who became devotees due to its pleasing interaction style and clinical value. Specifically, the compact device provides a numeric value for oxygen saturation (i.e., 98%). The numeric reading is complemented by a vertical colored bar that pulses up and down with each heartbeat, emitting a tone keyed to the patient's oxygenation level (the higher the pitch, the higher the patient's oxygenation level).
Needle-Stick-Prevention Devices. Shifting the focus away from microprocessor-based devices and toward hardware, needle-stick-prevention devices presented the development community with an interesting human factors challenge in the late 1990s. The challenge was to keep caregivers from infecting themselves with blood-borne diseases such as HIV due to needle sticks. Numerous inventors tackled the challenge, producing a bumper crop of needle-stick-prevention needles. Some of the designs are better than others at enabling caregivers to give injections or place a catheter without extra fuss. B. Braun Medical Inc.'s Introcan Safety Intraveneous Catheter is one of the best, garnering a Medical Design Excellence Award in 2003. After a single use, a metal fitting extends over the end of the needle to prevent a needle stick or an unauthorized second use. Human factors aficionados and nonspecialists alike admire the device's elegance in terms of its protective and unobtrusive characteristics. In addition, caregivers are able to continue to use their conventional methods of introducing catheters.
Automatic External Defibrillator. The Philips HeartStart Home Defibrillator has garnered several design awards for its compact form and usability. The device is designed for laypersons to operate in the event of a cardiac emergency. It uses extensive voice prompts to talk people through the delivery of cardiopulmonary resuscitation (CPR) as well as the delivery of a cardioverting shock. Significantly, the developers conducted extensive user-needs analyses and usability testing during the course of developing the product's widely admired user interface.
Patient Monitor. Many within the anesthesia community would agree that Datex Medical Instrumentation challenged the major players in the mid-1990s by introducing the AS/3 monitor. It was arguably the first integrated patient monitor designed specifically for use in the OR, as opposed to critical-care units. New users found it remarkably easy to use and well suited to the needs of anesthesia providers. For example, they liked the fact that waveforms were accompanied by large numeric readouts. They also appreciated the integration of gas-concentration measurements (e.g., O2, N2O, and anesthetic agents) along with hemodynamic measurements (e.g., respiratory rate, heart rate, and blood pressure). Though technical in nature, these enhancements reflected a sensitivity to the specific information needs of anesthesia providers in the OR versus nurses in critical-care units. Since the AS/3's introduction, Datex (now GE Medical Systems) has gained a significant portion of the U.S. market for integrated patient monitors.
Infusion Devices. The Alaris Medical Systems Medley Medication Safety System allows users to run multiple intravenous infusions via individual pumps connected to a central programming module. The system's defining characteristic is its Guardrails Safety Software that helps to protect patients from medication errors. Hospitals use the software to establish drug dose limits that caregivers may not exceed, thereby eliminating the potential of an overdose due to a data-entry error. The use of software to help protect users from making lethal errors heralds a new era of devices with built-in safety systems that extend beyond switch guards and alarms.
The Baxter Healthcare/Bard Infuse OR, a syringe pump, has been a favorite product among clinicians for many years. Introduced in 1988, the product simplified the task of determining a safe medication dose through the use of interlocking magnetic panels that attach to the product like a refrigerator magnet. The panels, which are uniquely tailored to specific medications, communicate electronically with the pump's microprocessor to ensure appropriate dosing schemes, which are reflected by the panel labeling.
Glucose Meters. First-generation glucose meters were a breakthrough in terms of giving diabetics the ability to measure their blood glucose levels on a daily basis. However, many models were compromised by the large number of steps in the test process, the considerable dexterity requirements, and the need for careful timing. The latest models, such as Lifescan's One-Touch Ultra, are impressively compact and require a few simple steps. The One-Touch Ultra presents the user with a result within 5 seconds via a large, easy-to-read display.
Looking Ahead
What lies ahead regarding the application of human factors engineering in the design of medical devices? The fundamental challenge remains to make human factors engineering one of the central considerations when developing medical devices that require substantial user interaction.
Even with the FDA quality system regulation in place, some companies still pay lip service to human factors engineering instead of making a meaningful investment in it. Fortunately, the marketplace is likely to correct this problem over time—possibly a long period of time—as customers come to reject hard-to-use offerings. In fact, some would argue that market demand, informed by user-interface-design excellence in other industries, is the real driving force toward better medical device user interfaces, as opposed to government regulation.
Aside from developing a design process that complies with FDA requirements, one of the biggest challenges for many device manufacturers will be smoothing the information management process. This is a challenge that extends beyond the design of a single device. The clinical environment is already awash in information generated by all sorts of devices, ranging from patient monitors, to infusion systems, to hospital beds, to wristbands that enable patient identification and tracking. Getting all of these disparate devices to work in harmony, particularly with regard to alarm messages, requires not only good design practice but also industry standardization so that they speak a common user-interaction language. The proprietary interests of various companies may create considerable hurdles to achieving this goal. In short, many manufacturers are committed to their brand of user interface and are loath to change. They want to avoid any added development expense and the loss of unique characteristics that serve to differentiate their products from others.
Goldman feels that devices need to get a lot smarter. He describes some of the alarms generated by devices such as patient monitors as totally unnecessary nuisances, noting that intelligent devices that adjust to the context of use would not be crying wolf. For example, Goldman says, “a patient monitor should communicate with anesthesia recordkeeping software to know when a patient is going on to cardiopulmonary bypass, and suppress the apnea alarm while activating other alarms in a context-sensitive manner.” As another example, Goldman presents the low-battery alarm. “It's fine for a device to alarm when there is 15 minutes of battery time remaining,” he says. “But after you acknowledge the first alarm, it should not alarm again every single minute.”
Goldman believes that the work that is under way in Massachusetts General Hospital's Operating Room of the Future will help pave the way toward the development and adoption of smarter medical technology that enhances information management. Currently, the R&D program is looking at several opportunities, including wireless communication between patient sensors and data collection devices.
Making medical devices easier to learn to use is another frontier. Today, an increasing number of devices have on-line help systems, tutorials, and simulators available that enable people to practice using them. However, caregivers still prefer hands-on training. Their preference can be attributed in part to tradition. The department in-service, complete with tasty refreshments, is a cultural norm that makes learning a shared experience rather than a solo adventure. But the limitations of in-service training—including the difficulty of reaching everyone working in a multishift, high-turnover institution—are well known. So human factors engineers and other specialists have their work cut out for them, striving to create effective and appealing educational resources. Advanced computer-based simulations that require substantial user interaction, making the experience feel quite hands-on, will clearly be in the mix.
One more frontier might be dubbed, “Back to the Future.” We may be experiencing the peak of physical estrangement from medical devices that feel more like computers than hands-on, physically interactive tools.
Caregivers have always liked direct manipulation: the ability to turn a knob or press a button. This is because the mechanisms are immediately accessible and provide tactile feedback in addition to visual and audible feedback. By comparison, selecting an on-screen icon, scrolling through a list of options, clicking on an “up” arrow, then clicking on an “enter” button, takes more time and effort. So look for a greater number of dedicated knobs and dials to balance out screen-based interactions.
Human factors engineers will also play an important part in ensuring the interactive qualities of complex medical devices used in the home, such as dialysis machines. Here, the key will be to create user interfaces that do not call for advanced medical and technological know-how, but rather lead users effectively through operating procedures as well as help them overcome problems that arise. Meeting this challenge is especially important given the pattern of migration of medical devices from clinical environments into the home and workplace.
Conclusion
You could say that human factors engineering in the medical domain has come a long way, emerging from a dark age of design negligence. But to be fully applicable, the tag line would have to read, “You've come a long way, baby...and you've got a ways to go.” Generally, manufacturers still do not regard human factors engineering as a fundamental need, equivalent to mechanical engineering or software programming. When a widespread changeover finally occurs, it will assuredly improve patient safety and reduce healthcare costs. Importantly, the added investment will raise the quality of life-critical medical devices to the level of the better kitchen tools and digital music players!
Still, the medical device industry has some shining examples of good human factors engineering, ranging from pocket-sized testing devices to large diagnostic scanners. These are winning design awards and gaining marketshare. They create a desire among device users for more devices of similar and even greater quality. So, the human factors movement in the medical industry is certainly heading in the right direction.
References
1.“The Report of the President's Commission on the Accident at Three Mile Island,” in Stellar-One.com [on-line], (Dover, AR: 30 October 1979); Available from Internet: www.stellar-one.com/nuclear/report_to_the_president.htm.
2.Steve Salvatore et al., “Study: Most New Doctors Can't Use Stethoscope,” in CNN.com [on-line] (Atlanta: 2 September 1997); Available from Internet: www.cnn.com/health/9707/02/nfm.
heart.sounds.
3.Code of Federal Regulations, 21 CFR 820.30.
4.“Why Is Human Factors Engineering Important for Medical Devices?” in CDRH Home Page [on-line] (Rockville, MD: FDA, Center for Devices and Radiological Health, 2003); Available from Internet: www.fda.gov/cdrh/humanfactors/important/html.
Copyright ©2004 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like






.png?width=300&auto=webp&quality=80&disable=upscale)
