A Practical Lesson from HeartWare's Latest Heart Pump Recall
February 23, 2015
What about medical devices used in clinical trials? It is a question that is apparently dogging HeartWare.
Chris Newmarker
|
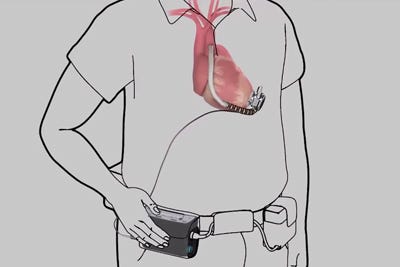
HeartWare's driveline cable, shown in this illustration from a company video, connects the implanted pump with an externally worn controller. |
HeartWare International recently announced that it was recalling older controllers used during 2012 clinical trials of its implantable heart pumps.
The older controllers, used by about 120 U.S. patients, exhibit a higher susceptibility to electrostatic discharge (ESD) than newer, commercial controllers, HeartWare (Framingham, MA) said in a Friday news release.
Such an ESD could cause the pump to stop, leading to serious injury or death, the company said. Since a voluntary Field Safety Corrective Action in 2013, HeartWare has received reports of one additional death and one additional serious injury in which ESD may have caused or contributed to a pump stop.
The latest recall does not apply to HeartWare's newer, commercial controllers because it has made design enhancements to them. The recall, then, provides a lesson of what can happen when not all of the design kinks have been figured out with a device before clinical trials.
The situation means that as soon as 2013, HeartWare was advising patients to reduce risk of ESD by avoiding such commonplace household activities as vacuuming and removing clothes from a dryer. They were also told they could avoid static electricity events by staying away from dry environments or certain fabrics and materials such as silk clothing and carpeting.
Now it is going as far as to advice clinicians to exchange the recalled controllers under medical supervision with a new controller, when advisable.
The latest recalls comes about a year after news of a recall involving a faulty driveline connector that could cause the heart pump to temporarily stop.
Refresh your medical device industry knowledge at BIOMEDevice Boston, May 6-7, 2015. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like