PMA Approvals Hit 15-Year High
FDA's PMA approval rate was 98% in the most recent fiscal year. The agency hopes to further improve device approval timelines while simultaneously safeguarding patients.
Brian Buntz and Chris Newmarker
|
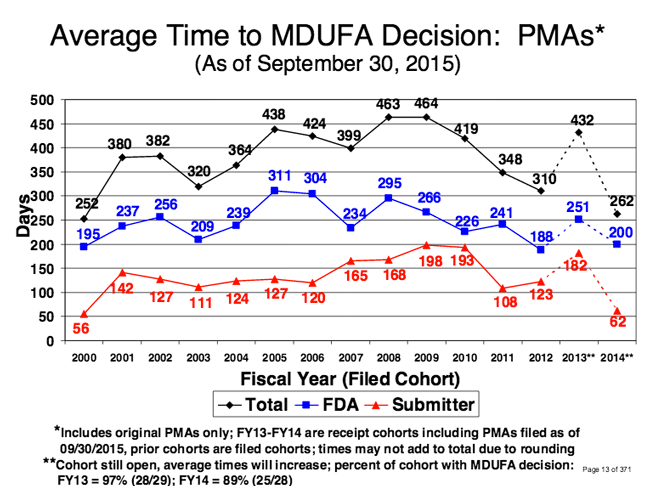
PMA approvals are now substantially quicker than they were in 2008 and 2009. Image drawn from an FDA report. |
The FDA has approved 98% of PMA applications during its most recent fiscal year ended September 30, marking the highest approval rate in at least 15 years and the first time the approval rate has been 90% or above since 2005.
The percentage has been on the rise over the past five years. From a low point of 59% in 2010, it rose to 70% in 2011, 71% in 2012, 85% in 2013, and 86% in 2014, according to a recent FDA report.
The statistics suggest that FDA is trying to make good on its promise to get cutting-edge medical technologies to U.S. residents as quickly as possible. CDRH director Jeffrey Shuren, MD, even went as far as to say in October that he would like the U.S. to be getting new medical technologies to patients faster than any other country in the world--presuming the agency can maintain its standards in doing so, he stressed.
"This is not a competition. We are not in a race with Europe or anywhere else. But if there is good technology, we do want to have it come out first," Shuren said at the Transcatheter Cardiovascular Therapeutics conference in San Francisco.
Not only are PMA approval percentages up, but the agency has been working to accelerate its time to decision for PMAs as part of its ongoing user fee acts with the medical device industry--most recently MDUFA III.
Most PMAs are subject to user fees, and the FDA often does not start the review clock until it has received payment.
Since 2009, the average time to has decreased. In 2009, the average time to decision for PMAs was 464 days; in 2014, it was 262 days--a 31% reduction within five years. For submissions the FDA received in the fiscal year 2015, the average time was390 days.
The average time to clear 510(k) submissions has also been trending downwards, from a 2010 peak of 154 days to a projected 95 days in 2015. The percent of 510(k)s with additional information requested has also been dropping in recent years. For 2015, 69% needed additional information during the first review cycle, and only 6% needed additional information during the second review cycle.
The 510(k) process is used to clear the vast majority of non-Class I medical devices whereas PMAs account for roughly 1% of device submissions to FDA.
Some in Congress and elsewhere have expressed concerned that FDA is sacrificing safety as it seeks to accelerate innovation and have specifically called into question the agency's handling of its 510(k) premarket notification process. For example, U.S. Sens. Jeff Merkley, D-OR, and Edward Markey, D-MA, recently sent a letter to the FDA expressing concern about the "weakness in the premarket review process used to evaluate most medical devices."
Indeed, medical device recalls have also been trending up over the course of the past decade. According to a FDA report released in 2014, medical device recalls had increased nearly 100% from 2003 to 2012. The agency attributed the increase to "enhanced awareness by device firms, including those that were cited for reporting violations; and specific CDRH efforts to improve medical device safety." In other words, FDA is saying the recalls are up because the agency and the industry are doing a better job at identifying problems.
Learn more about cutting-edge medical devices at MD&M West, February 9-11 at the Anaheim Convention Center in Anaheim, CA. |
Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz.Chris Newmarker is senior editor of MPMN and Qmed. Follow him on Twitter at @newmarker
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like