Packaging and Sterilization Wrap-Up
March 21, 2005
Originally Published MPMN March 2005
PRODUCT UPDATE
Packaging and Sterilization Wrap-Up
Susan Wallace
|
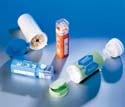
Ultraclean nylon bags from Degage Corp. are suitable for the critical packaging needs of medical and electronic parts. |
Medical device packagers have a very important job. The best device in the world won’t save any lives until it’s in the hands of a healthcare professional. And it can’t get there safely unless it’sbeen properly packaged. It also may do harm unless it’s been sterilized so that it will not introduce any contaminants into the patient.
Following is a roundup of some of the latest innovations in the medical device packaging and sterilization industries. .
Medical-Grade Nylon Packaging Available for Critical Devices
Ultraclean nylon bags and films are available for the packaging of critical medical and electronic parts. The nylon and antistatic bags from Degage Corp. (Terrell, TX) are fabricated in a vertically integrated, federally certified 209e cleanroom. Applications include medical instruments, orthopedic devices, spinal products, and neurosurgical devices, as well as ceramics, thin films, and fiber optics.
The company also manufactures thin-gauge, narrow-width, zero-tolerance tubing for the packaging of catheters and other critical medical devices. Its Clean-Tuff film is as strong in 2 mil as most 4-mil films, according to the firm. The company’s vertical integration allows it to achieve shipping times of 2 weeks.
Polymer Packaging Can Be Customized
|
Sud-Chemie’s polymer packaging solutions can |
A company’s polymer packaging can be molded into virtually any shape or size. The line from Sud-Chemie Performance Packaging (Belen, NM) includes both standard and customized products such as tubes with a dessiccant stopper, flip-top tubes for diagnostic test strips, dosers, and dispensers.
Another type of packaging, the 2AP dessiccant polymer, offers advanced moisture protection, enabling moisture-sensitive components such as the caps of fertility-test kits or complete IVD housings to be molded from dessiccant material. Also by using the polymers, manufacturers can streamline workflow by eliminating the need for dessiccant insertion, foil pouching, or carton packaging.
The company can print important information on the polymer packages in up to six colors. Unlike tubes that are injection molded with integral lids, Sud-Chemie’s flip-top tubes are made in two separate pieces. This allows for easy printing of the tubes prior to assembling the flip-top lids.
Manufacturers can use the polymer products in their high-speed packaging or filling lines. For instance, the company’s flip-top tubes feature a hinge design that allows the hinge to be removed and reattached without altering the functionality of the product.
Materials Supplier Offers New Packaging Capabilities
A company provides two new packaging services. Packaging size customization from Nusil Technology (Carpinteria, CA) enables customers to dispense and discard process-specific packaging. This eliminates waste. Submicron filtration helps lower costs by improving user efficiencies.
“Specialized, ready-to-use packaging has multiple benefits to multiple industries, even more so when the technology is customizable for specific applications,” says Richard Compton, the company’s cofounder and CEO. “With dedicated space and increased expertise in final processing and packaging, our in-house team of material experts can engineer custom packaging solutions based on each customer’s unique property requirements.”
Multiple Sterilization Facilities Minimize Transportation Costs
|
Steris Isomedix Services offers comprehensive contract sterilization services to the medical device industry. |
A company offers gamma, EtO, and E-beam sterilization services. Steris Isomedix Services (Mentor, OH) has a keen understanding of supply chain management, and works with its customers to improve turnaround time, reduce inventory, and enhance time to market. It has a network of 16 North American facilities to help achieve this goal.
Gamma processing using dosimetric release protocol enables products to be shipped immediately after sterilization. This can be done in 24 to 48 hours or less, from processing to outbound shipping. This method is suitable for single-use medical supplies such as syringes, catheters, IV sets, gloves, and face masks.
Another company, Centurion Sterilization Services (Howell, MI), has three facilities to handle logistics for every region in the country. On-site full-service laboratories allow samples to be tested immediately. This eliminates extra expense or transportation delays. Only medical devices are tested, no powders, talc, or seeds.
Copyright ©2005 Medical Product Manufacturing News
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
