Outsourcing Outlook on Packaging and Sterilization Services
June 25, 2014
Clipless dispenser
 In an effort to reduce packaging waste, Cleancut Technologies Inc. offers the patented clipless guidewire dispenser. Manufactured using a thermal bonding process, the dispenser is a solid unit that is free of extraneous clips, which can puncture pouches, shift in transit, or fall off. clip failure can result in skewed lumens, potentially damaging or kinking the products within the package. Aesthetically pleasing and structurally sound, the clipless and adhesive-free dispenser features a low profile, can be stored flat, and utilizes space more efficiently than other packaging designs. An FDA-compliant and ISO 13485-certified medical device packaging company, the service provider manufactures packaging in class 7 and class 8 environments with full traceability.
In an effort to reduce packaging waste, Cleancut Technologies Inc. offers the patented clipless guidewire dispenser. Manufactured using a thermal bonding process, the dispenser is a solid unit that is free of extraneous clips, which can puncture pouches, shift in transit, or fall off. clip failure can result in skewed lumens, potentially damaging or kinking the products within the package. Aesthetically pleasing and structurally sound, the clipless and adhesive-free dispenser features a low profile, can be stored flat, and utilizes space more efficiently than other packaging designs. An FDA-compliant and ISO 13485-certified medical device packaging company, the service provider manufactures packaging in class 7 and class 8 environments with full traceability.
Cleancut Technologies Inc.
Anaheim, CA
www.cleancuttek.com
Electromedical device packaging
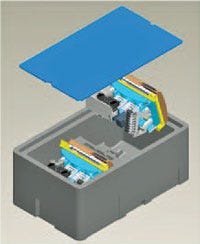 Sonoco Protective Solutions is involved in the design of custom electromechanical medical product packaging. Typically this type of protective packaging is complex and expensive. Even when producing low volumes (250 to 750 units per year), the company can introduce molded EPP foam in combination with traditional wood and corrugated materials, reducing packaging costs by 25 to 35%. It accomplishes this by redesigning, prototyping, and then testing the product in its ISTA certified lab. In addition, the company's Transguard product can be used for hemodialysis components, injection-molded assemblies for blood oxygenators, and injection molded final packaging. for such applications, the Transgaurd product replaces typical corrugated expendable packaging.
Sonoco Protective Solutions is involved in the design of custom electromechanical medical product packaging. Typically this type of protective packaging is complex and expensive. Even when producing low volumes (250 to 750 units per year), the company can introduce molded EPP foam in combination with traditional wood and corrugated materials, reducing packaging costs by 25 to 35%. It accomplishes this by redesigning, prototyping, and then testing the product in its ISTA certified lab. In addition, the company's Transguard product can be used for hemodialysis components, injection-molded assemblies for blood oxygenators, and injection molded final packaging. for such applications, the Transgaurd product replaces typical corrugated expendable packaging.
Sonoco Protective Solutions
Arlington Heights, IL
www.sonoco.com
Components repackaging
 IS Med Specialties (ISM) has debuted the ability to repackage medical components. The repackaging capability includes volume reduction from a large quantity bag to smaller bags and multipart kitting into a single bag. The company uses an class 8 cleanroom to repackage medical components without introducing additional particulate matter or bioburden. In addition to its repackaging services, it provides custom medical assembly services under cleanroom conditions. Services offered include the ability to cut custom tubing, fit tubing to fitting connections, and produce part-to-part assemblies. The ISO 9001:2008-certified firm also offers custom design support through the use of 3-D CAD technology.
IS Med Specialties (ISM) has debuted the ability to repackage medical components. The repackaging capability includes volume reduction from a large quantity bag to smaller bags and multipart kitting into a single bag. The company uses an class 8 cleanroom to repackage medical components without introducing additional particulate matter or bioburden. In addition to its repackaging services, it provides custom medical assembly services under cleanroom conditions. Services offered include the ability to cut custom tubing, fit tubing to fitting connections, and produce part-to-part assemblies. The ISO 9001:2008-certified firm also offers custom design support through the use of 3-D CAD technology.
IS Med Specialties
Englewood, CO
www.ismedspec.com
NO2sterilization services
 Noxilizer Inc. provides medical device manufacturers with contract sterilization services based on nitrogen dioxide (NO2) technology. Its proprietary room- temperature NO2 sterilization technology compares favorably with traditional sterilization methods using EtO, gamma irradiation, and hydrogen peroxide in terms of safety and the length of processing cycles. The standard cycle lasts 60 to 90 minutes and features immediate release. NO2 sterilization maintains material properties, requires no additional aeration, leaves no cytotoxic residuals, and is compatible with such combination products as prefilled syringes, collagen scaffolds, and bioresorbable implants.
Noxilizer Inc. provides medical device manufacturers with contract sterilization services based on nitrogen dioxide (NO2) technology. Its proprietary room- temperature NO2 sterilization technology compares favorably with traditional sterilization methods using EtO, gamma irradiation, and hydrogen peroxide in terms of safety and the length of processing cycles. The standard cycle lasts 60 to 90 minutes and features immediate release. NO2 sterilization maintains material properties, requires no additional aeration, leaves no cytotoxic residuals, and is compatible with such combination products as prefilled syringes, collagen scaffolds, and bioresorbable implants.
Noxilizer Inc.
Baltimore
www.noxilizer.com
Frangible-seal technology
 With an engineering focus and 30 years of expertise, J-Pac Medical provides design, prototype development, qc validation, manufacturing, and supply chain services to support medical device and diagnostic companies. Ongoing capital investments have allowed the company to provide frangible-seal technology for diagnostic point-of-care applications. It has also been investing in the area of surgical implants, enabling it to broaden its expertise in textiles, including 3-D forming, edge treatments, and particulate-free cutting. J-Pac Medical
With an engineering focus and 30 years of expertise, J-Pac Medical provides design, prototype development, qc validation, manufacturing, and supply chain services to support medical device and diagnostic companies. Ongoing capital investments have allowed the company to provide frangible-seal technology for diagnostic point-of-care applications. It has also been investing in the area of surgical implants, enabling it to broaden its expertise in textiles, including 3-D forming, edge treatments, and particulate-free cutting. J-Pac Medical
Somersworth, NJ
www.j-pac.com
About the Author(s)
You May Also Like


