New Rigid Medical Packaging Material Good for Device Makers and Thermoformers
Created through a team effort between an extruder, chemical company, and thermoformer, a new medical packaging foam offers strong protection, clean processing, and a styrene-free alternative.
July 6, 2015
Created through a team effort between an extruder, chemical company, and thermoformer, a new medical packaging foam offers strong protection, clean processing, and a styrene-free alternative.
Marie Thibault
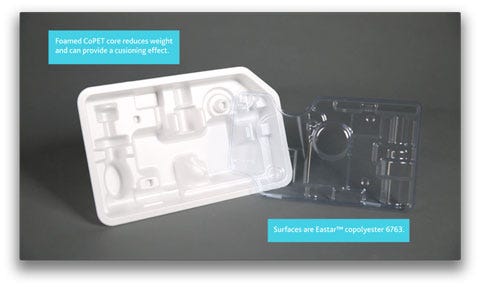
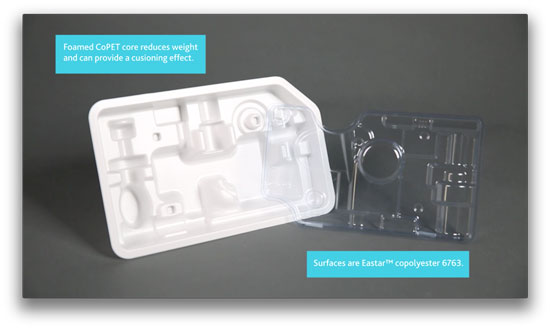
An example of rigid medical packaging made with Pacur PETG foam, in a still from a video provided by Eastman Chemical Company.
When most people think of "innovation" in the medical industry, they probably think of the latest improvements to make medical devices smaller, with longer batter lives, and wireless connectivity. But innovation comes into play when it comes to protecting these medical devices during shipping too.
Three companies—Pacur, LLC, Eastman Chemical Company, and Tek Pak, Inc.—worked together to develop the new Pacur PETG foam. This foam, which boasts a light weight yet offers reliable protection against bumps during shipping, is made with Eastman Eastalite copolyester for its core and Eastman Eastar copolyester 6763 for the outside "skin" layers. Tek Pak processed the new material.
Representatives from all three companies—Aneta Clark, market development manager at Eastman; James Banko, vice president of sales at Pacur; and Tony Beyer, president of Tek Pak—sat down with MD+DI at the MD&M East Conference in New York City in June to discuss the new packaging material. They explain that the foam is a team effort, with the pellets supplied by Eastman, extrusion performed by Pacur, and thermoforming done by Tek Pak.
Together, Clark, Banko, and Beyer point out the advantages of the material. Beyer, from thermoformer Tek Pak, notes that Pacur PETG foam cuts cleanly, creating very little particulate and making the material a good choice for clean rooms. In an Eastman press release announcing the new material, Beyer said, "We . . . found it to be cleaner when looking at angel hair and particulate generation. The material is easily trimmed and removed from the forming machine and has a beautiful pearlescent look . . ."
There are other benefits for thermoformers, Beyer says. He points to the example package—a thermoformed rigid tray—that was designed to test the material for trouble spots during thermoforming. Difficult areas include undercuts—Beyer describes them as negative formations—tall, thin walls, stacked shoulders, and intricate details. The tray, seen in the photo above, includes all these challenges. The Pacur PETG foam performed well against all these hurdles and in addition, the material runs faster than styrene because it requires less heat, Beyer says.
The experts point out a couple other benefits. The edges of the material aren't as sharp as HIPs, which should help prevent punctures and keep devices sterile during shipping. The material maintains its capabilities following ethylene oxide sterilization or gamma irradiation, standing up to a 15-pound drop test in a promotional video.
In addition, the foam is styrene-free, making it a sustainable choice. In the Eastman press release, Clark said, "Eastman Eastalite copolyester resonates among processors and thermoformers because of its ease of processing and clean cutting . . . This is extremely important to us because it allows our value chain collaborators to gain efficiencey in their prouction and even cut out some secondary processes . . ."
Banko, from Pacur, says the new offering is a result of listening to industry's request for an alterative to High Impact Polystyrene (HIPS). He says the company is already speaking with original equipment manufacturers (OEMs) about the recently-launched material.
Enhance your medtech knowledge by attending MEDevice San Diego, September 1–2, 2015, in San Diego. |
Marie Thibault is the associate editor at MD+DI. Reach her at [email protected] and on Twitter @medtechmarie.
[Image courtesy of EASTMAN CHEMICAL COMPANY]
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
